FDA Approval of Whooping Cough Vaccine for Infants Less Than 2 Months of Age
Digital Pharmacist
OCTOBER 26, 2022
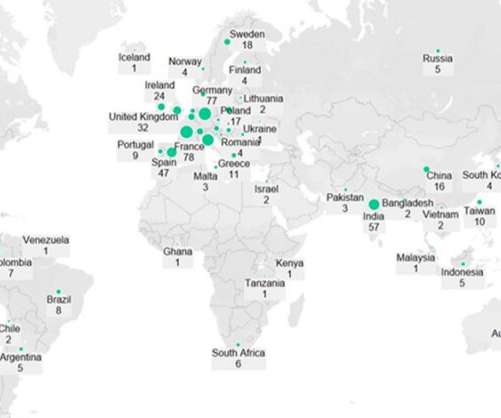
On October 7 th , 2022 the U.S. Children aged 2 months of age or younger are not eligible for the Tdap vaccine. The lack of solid data around the effectiveness and safety of the Tdap vaccine on the newborn itself was what led to further research. The FDA considered the safety data with the non-U.S. Background of Boostrix.




























Let's personalize your content