Documentation Pays Off in More Than Way Than One
Drug Topics
OCTOBER 5, 2022
Quality documentation ensures that pharmacies get paid for clinical services and offers a way to engage with patients.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
 Documentation Related Topics
Documentation Related Topics 
Drug Topics
OCTOBER 5, 2022
Quality documentation ensures that pharmacies get paid for clinical services and offers a way to engage with patients.

Drug Topics
SEPTEMBER 17, 2022
But is the collected information being used to help with documentation, care planning, and reimbursement? Big data and analytics are everywhere—including in independent pharmacy.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Drug Topics
JANUARY 10, 2024
The estimated efficacy of the vaccine against documented infection for children and adolescents during the Omicron wave was 74.3% and 85.5%, respectively.

Drug Topics
MARCH 22, 2024
Researchers aimed to address the adverse effects of veterans with COVID-19 diagnoses 18 months after infections were documented.

The FDA Law Blog
APRIL 8, 2025
Indeed, since 2014, DPD facilitatedthe publication of 42 quarterly batches and dozens of stand-alone PSGs, plus three one-off batches of PSGs updated to align with recommendations in general guidance documents ( g. , Under GDUFA III alone, DPD published 32 GDUFA guidance documents and MAPPs to ensure the successful implementation of GDUFA.

Fierce Healthcare
FEBRUARY 22, 2024
. | Along with the funding round, Abridge also announced an enterprise agreement with Connecticut-based Yale New Haven Health System to give thousands of clinicians access to its AI-powered clinical documentation technology.

STAT
FEBRUARY 3, 2024
Documents released this week shed new light on an aggressive strategy from vape maker Juul to court Black leaders, including the Rev. Al Sharpton, to publicly support its e-cigarettes. It’s not clear how much the company ultimately spent on the partnerships. Continue to STAT+ to read the full story…

Fierce Pharma
SEPTEMBER 19, 2024
From truckloads of torn documents to avian incursions in the production plant, Granules India’s recent manufacturing reprimand from the FDA is alarming no matter which way you look at it. Earlier this month, Granules was slapped with a Form 483 following an inspection of its Telangana facility in India that ran from Aug. 26 to Sept.
STAT
APRIL 24, 2024
HCA Healthcare, the largest for-profit hospital chain in the United States, is planning to expand the use of an artificial intelligence tool to document doctor-patient interactions in its emergency rooms.

STAT
JANUARY 18, 2024
Microsoft on Thursday said it will launch its artificial intelligence tool for automating clinical documentation within health records software made by Epic, a move to embed the technology in health systems nationwide.

STAT
NOVEMBER 15, 2022
However, a STAT review of documents detailing the steps taken toward government approval found that regulators endorsed the vaccine, called Covaxin, despite discrepancies in the number of clinical trial participants.

Fierce Pharma
JUNE 7, 2023
On Wednesday, that vision came into better focus as the FDA released a document showing how the agency views the drug. . | As Alzheimer's partners Eisai and Biogen lay the groundwork for a wider launch of Leqembi, their efforts center on winning a full FDA approval.

STAT
SEPTEMBER 12, 2023
WASHINGTON — There’s a panel of 20 nutrition experts that has outsized influence on the American diet — and the food industry has worked hard to get friendly researchers into the group, new documents obtained by STAT show.

The FDA Law Blog
APRIL 21, 2025
By John W.M. Claud & Michelle L. Butler Recent reductions in force (RIFs) and leadership changes at FDA are already affecting key agency functionsand as the administration plans a broader reorganization, the impact will likely grow. One area drawing increasing attention is how these changes will affect the drug development and review process.

STAT
SEPTEMBER 19, 2024
Specifically, a “large number of torn pieces” of documents that should have been kept to verify manufacturing and testing practices were found in the trucks, as well as in a scrap bag at a site run by Granules in Telangana, India.

STAT
MAY 1, 2024
In study documents, Mount Sinai doctors said the biopsies result in “the same amount of tissue loss” and “in effect, the same level of risk” for patients as standard DBS, because they are removing tissue that would otherwise be cauterized.
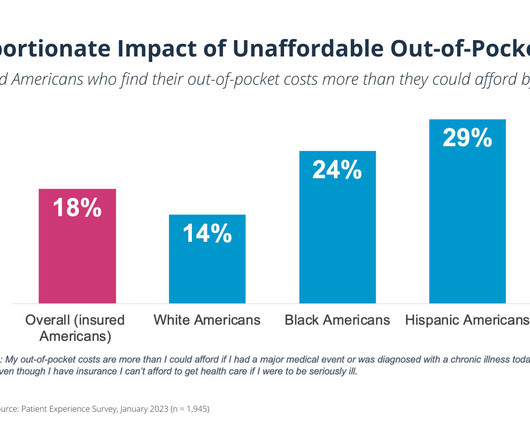
PhRMA
MARCH 29, 2023
This series – including surveys of 5,000 Americans conducted with Ipsos, a leading research company in the United States — documents a consistent trend in reports of insurer- and middlemen-imposed practices that can keep patients from the medicines and treatments that they need.
STAT
JANUARY 7, 2025
The advent of the first generic GLP-1 drugs could help Medicare negotiate a lower price for the highly sought after diabetes and obesity medication semaglutide , according to experts familiar with the price-negotiation program and STAT’s review of documents from the first round of negotiations.

Fierce Healthcare
FEBRUARY 20, 2024
MA governor orders Steward Health Care to disclose withheld financial documents, says it must exit state 'as soon as possible' dmuoio Tue, 02/20/2024 - 16:37

STAT
APRIL 13, 2025
In an unusual move, an audit of commercial health plans by Tennessee officials found that Express Scripts, one of the largest pharmacy benefit managers in the United States, violated state laws in its dealings with pharmacies, according to newly released documents.

Fierce Healthcare
MARCH 8, 2024
WellSpan Health is taking the burden off its providers by deploying an AI-driven clinical documentation tool from Nuance. | DAX Copilot, from Microsoft’s speech recognition subsidiary Nuance Communications, integrates with Epic to draft clinical notes during visits.

Fierce Healthcare
OCTOBER 16, 2024
Microsoft recently unveiled several new artificial intelligence capabilities for healthcare organizations designed to help analyze diverse medical data, streamline nursing documentation and enable | Microsoft recently unveiled several new AI capabilities for healthcare organizations designed to help analyze diverse medical data, streamline nursing (..)

STAT
MARCH 7, 2025
UnitedHealth acquired full or partial ownership stakes in more than 100 surgery centers in 2024, according to a STAT review of UnitedHealth’s newest annual financial documents.

STAT
JULY 15, 2024
WASHINGTON – Top Food and Drug Administration officials met multiple times earlier this year to discuss the regulation of ultra-processed foods, according to internal agency calendars obtained by STAT.

Hospital Pharmacy Europe
JULY 1, 2025
In response to this, the Leeds Teaching Hospitals NHS Trust (LTHT) pharmacy team has recently launched its ‘Digital Induction’ document. Initial feedback has been overwhelmingly positive, leading to the creation of additional documents tailored for line managers and pharmacy students with specific digital needs.
Drug Topics
JANUARY 16, 2023
In the 3 cases documented by researchers, hepatitis B surface antigen (HBsAG) levels were reduced by more than 50% after Japan began its COVID-19 vaccination program.

STAT
MAY 6, 2024
WASHINGTON – Two New York University professors collaborated directly with executives of the vaping company Juul without disclosing those relationships to academic journals or Congress, a STAT investigation reveals.

Fierce Healthcare
MAY 22, 2024
A wave of employee layoffs that UPMC announced last month was just one part of a broader restructure and operations “transformation” initiative, for which the nonprofit system has tapped management | An internal presentation obtained by Fierce Healthcare outlined April's “tough, but necessary" layoffs as one part of a broader look at the (..)
STAT
JANUARY 29, 2024
LONDON — There was something odd about these Alzheimer’s cases. Part of it was the patients’ presentations: Some didn’t have the classic symptoms of the condition. But it was also that the patients were in their 40s and 50s, even their 30s, far younger than people who normally develop the disease.

IDStewardship
MARCH 17, 2024
Ctrl+F, but better Believe it or not, the reliable Ctrl+F command, cherished for bringing simplicity to document-searching, has been eclipsed by the advancements of AI. Ctrl+F can struggle with vague or common terms, making it challenging to find specific information in dense documents. No problem.
STAT
DECEMBER 30, 2024
The authors found references to skin tone, pigmentation, or race in 4% of the documents submitted before the FDA made its suggestion to diversify skin tone testing in 2013.
STAT
OCTOBER 27, 2023
The Food and Drug Administration said on Friday that it has some safety concerns about an experimental CRISPR-based treatment for sickle cell disease, citing the methods used by its makers to evaluate the risk of inadvertently making unwanted changes to patients’ DNA.

Drug Topics
MARCH 24, 2024
At this year’s American Pharmacists Association Annual Meeting & Exposition, Mark Garofoli urged pharmacists to develop strong documentation practices and go beyond baseline federal regulations to care for patients with red flags.

Drug Topics
JUNE 11, 2025
This led researchers to explore a specific region of the world in order to better document patients’ understanding of the disease. The prevalence of psoriasis in the Gulf countries is not well-documented; however, recent studies suggested that psoriasis affects 0.5%-5.3%

Drug Topics
JUNE 11, 2025
The study cohort included 670 patients who were diagnosed with PsA of at least a 3 month duration and have active plaque psoriatic skin lesions or a documented medical history of plaque psoriasis. READ MORE: Significant Knowledge Gap Exists in Patient Awareness of Psoriatic Disease The study found that 54.2%

STAT
JUNE 9, 2025
Get your daily dose of health and medicine every weekday with STAT’s free newsletter Morning Rounds. Sign up here. And another week begins — I kind of cannot believe that we’re already nearing “mid” June.

STAT
MAY 31, 2024
More than three years ago, the National Institutes of Health launched a $1 billion-plus initiative to find the root causes and potential treatments for long Covid , the chronic disease that has quickly changed the lives of millions of Americans.

The FDA Law Blog
JANUARY 9, 2025
Here, the FDA investigators documented several instances in which the Quality Manager appeared directly responsible for preventing company employees from talking with FDA about their responsibilities or responding to direct questions from FDA about areas in which FDA has authority to inspect. FDA Guidance , at 8 (emphasis added).

Pharmacy Times
JUNE 24, 2022
The impact of the pandemic on the mental health of health care workers, including pharmacists and pharmacy staff, has been well documented.

STAT
JUNE 27, 2025
And so Hassan called on GSK to return the product to market and, meanwhile, asked for numerous documents concerning pricing, rebates and communications with regulators. This marks the second time in recent months that GSK has angered a lawmaker over the asthma inhaler switch.

STAT
MARCH 20, 2024
Johnson & Johnson has accused a long-standing employee of taking thousands of confidential files about commercial strategies as he left for a similar job at Pfizer, according to lawsuit filed in a federal court in New Jersey.

European Pharmaceutical Review
FEBRUARY 3, 2025
The document identified significant violations of current good manufacturing practice (cGMP) regulations for finished pharmaceuticals. Inadequate validation of test methods : KVK-Tech failed to establish and document the accuracy, sensitivity, specificity and reproducibility of test methods.
Pharmacy Times
JUNE 10, 2025
Low uptake of vaccination to protect against invasive pneumococcal disease (IPD) was observed both before and after a pneumococcal-related hospitalization, with countless missed vaccination opportunities documented.
Pharmacy Times
SEPTEMBER 13, 2024
In the absence of a common treatment for long COVID, researchers from around the world documented the best currently available therapies.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content