Drugflation Is Soaring, But It Can Be Tamed
Pharmacy Times
JULY 26, 2024
Unbridled drugflation, or a point-in-time measure of the rising cost of pharmacy benefits, is taking its toll on corporate bottom lines and household budgets.

Pharmacy Times
JULY 26, 2024
Unbridled drugflation, or a point-in-time measure of the rising cost of pharmacy benefits, is taking its toll on corporate bottom lines and household budgets.
STAT
JULY 26, 2024
Want to stay on top of health news? Sign up to get our Morning Rounds newsletter in your inbox. I’m Brittany Trang, STAT health tech reporter and your new Friday morning host.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Drug Topics
JULY 26, 2024
Researchers aimed to address the associations between prenatal cannabis use and maternal health outcomes over a 9-year period.

STAT
JULY 26, 2024
A decade ago, Pfizer began investing heavily in gene therapy, bringing in experimental treatments for a range of genetic diseases, pumping $800 million into “state-of-the-art” manufacturing facilities and announcing its intention to become an “industry leader” that would deliver “one-time, transformative therapies” for rare diseases.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.

Drug Topics
JULY 26, 2024
Check out these important FDA updates from the month of July 2024.

Fierce Pharma
JULY 26, 2024
Johnson & Johnson’s latest attempt to get a handle on its talc litigation using the controversial Texas two-step bankruptcy strategy has once again fallen flat after a federal appeals court rul | The ruling from the Third Circuit Court of Appeals came the night before claimants in the talc litigation were set to vote on a sweeping $6.48 billion settlement proposal from J&J.
Pharmacy Technician Pulse brings together the best content for pharmacy technicians from the widest variety of industry thought leaders.

Fierce Pharma
JULY 26, 2024
Amid the sturm und drang over Inflation Reduction Act (IRA) drug price negotiations, Bristol Myers Squibb has been among the biopharma companies casting the most woe over the oncoming measures. | Bristol Myers Squibb CEO Chris Boerner said after seeing the final negotiated price for Eliquis that he is "increasingly confident" the company can navigate the impact of the IRA.

STAT
JULY 26, 2024
LONDON — European regulators on Friday said that an Alzheimer’s therapy from Eisai and Biogen should be rejected, again diverging from their U.S. counterparts on a medicine for a condition where treatments are desperately needed. In a statement, the regulators said that the benefits of Leqembi did not outweigh the risks of potentially dangerous side effects.
Pharmacy Times
JULY 26, 2024
The investigational drug achieved non-inferiority compared to routine Factor VIII.

STAT
JULY 26, 2024
Want to stay on top of the science and politics driving biotech today? Sign up to get our biotech newsletter in your inbox. Good morning. We’ve made it to the end of the week! I don’t know about you, but it’s felt like an especially long week for me. It’s probably cause of all the news that we will get into right now.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

Pharmacy Times
JULY 26, 2024
The molecule can be a potential treatment that counters effects of early-life antibiotics, which can impact the gut microbiota and increase the risk of childhood atopy and asthma.

STAT
JULY 26, 2024
On July 6, 2024, Sonya Massey, 36, called 911 to report a potential home intruder at her home in Springfield, Ill. “Don’t hurt me,” were her first words to the two officers who responded. Deputy Sean Grayson reassured her, “Why would I hurt you? You called us.” When Massey was questioned about her mental well-being, she confirmed taking her medicine.

Pharmacy Times
JULY 26, 2024
Medical professionals describe how pneumococcal disease manifests in adults, detailing its potentially lethal consequences such as sepsis or severe pneumonia, and explain how vaccination can effectively mitigate these serious health risks.

STAT
JULY 26, 2024
About 5% of Americans require skilled care at some point as they age. The horrific reports of more than 200,000 deaths of nursing home residents and staff during the Covid-19 pandemic put the nursing home industry under intense national scrutiny. But not all nursing homes experienced this level of tragedy and loss. One major reason why some fared better than others was adequate nurse staffing.

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.

pharmaphorum
JULY 26, 2024
Vaccination with GSK's recombinant shingles vaccine Shingrix could delay the onset of dementia, according to researchers in the UK.A study published in Nature Medicine suggests that people administered Shingrix have a lower risk of dementia compared to those given Zostavax - an older shingles shot based on a different technology – and builds on earlier research suggesting a benefit from this type of vaccination.

STAT
JULY 26, 2024
“Regulation is stifling innovation” seems to be a prevailing opinion among medtech leaders who believe the Food and Drug Administration’s rules are slowing medical device advancements — especially when it comes to software. I couldn’t disagree more. As someone who has led artificial intelligence (AI) and machine learning (ML) efforts at Amgen, I’ve seen firsthand how vital these regulations are for keeping patients safe.
Pharmafile
JULY 26, 2024
Clinical-stage biopharmaceutical company AC Immune announced that the US Food and Drug Administration (FDA) has granted its Alzheimer’s disease (AD) candidate ACI-35.030 (now called JNJ-2056) Fast Track designation. ACI-35.030 is an active-immunotherapy targeting phosphorylated Tau (pTau). It has been shown to induce a strong polyclonal antibody response against Tau aggregation, thereby potentially reducing the pathological […] The post AC Immune’s pTau-targeting immunotherapy awarded Fast

Pharmacy Times
JULY 26, 2024
The FDA’s designation builds off positive results in a phase 1b/2a clinical trial, showing that the vaccine can effectively target the Tau protein.

pharmaphorum
JULY 26, 2024
CHMP recommends against approval of Eisai, Biogen's Alzheimer's disease therapy Leqembi, saying side effects outweigh its benefits

Pharmacy Times
JULY 26, 2024
Understanding preventive care reduces the risk of illnesses and decreases future costs.

STAT
JULY 26, 2024
Hired someone new and exciting? Promoted a rising star? Finally solved that hard-to-fill spot? Share the news with us, and we’ll share it with others. That’s right. Send us your changes, and we’ll find a home for them. Don’t be shy. Everyone wants to know who is coming and going. And here is our regular feature in which we highlight a different person each week.

Fierce Pharma
JULY 26, 2024
After Eli Lilly’s Olumiant won the distinction of becoming the first med approved by the FDA to treat the follicle-attacking autoimmune disease alopecia areata, Pfizer followed up with a green ligh | The FDA signed off on Sun’s oral JAK inhibitor deuruxolitinib to treat adults with severe alopecia areata. The drug is now approved in 8 mg tablets under the brand name Leqselvi.
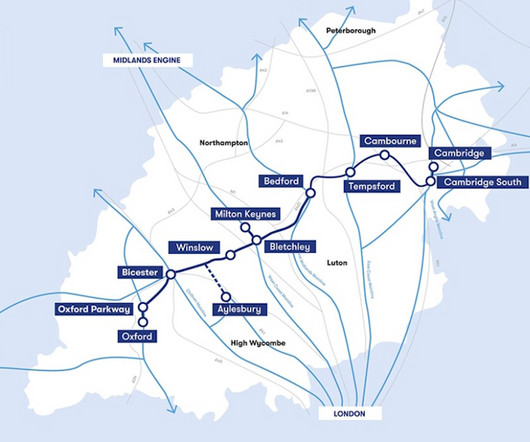
pharmaphorum
JULY 26, 2024
Leading figures from industry and academia, including AstraZeneca, have urged UK PM Keir Starmer to turn the Oxford-Cambridge region into the "crown jewel" of his industrial growth plan

Pharmafile
JULY 26, 2024
Calliditas Therapeutics AB today announced that the phase 2b TRANSFORM trial met its primary endpoint. The trial showed statistically significant improvement in alkaline phosphatase (ALP) for both doses tested versus placebo. The trial evaluated setanaxib, a NOX enzyme inhibitor, in patients with primary biliary cholangitis (PBC) and elevated liver stiffness.

Pharmacy Times
JULY 26, 2024
Medical experts outline the key indicators, symptoms, and transmission mechanisms of pneumococcal infections in adult populations.

PharmaVoice
JULY 26, 2024
Citius Pharmaceuticals CEO Leonard Mazur has personally invested $22.5 million into the company, which is fast approaching a potential turning point.

Fierce Pharma
JULY 26, 2024
While it may well be the dog days of summer, European drug regulators are showing no signs of slowing down in their review of new medicines and proposed label expansions. | While it may well be the dog days of summer, European drug regulators are showing no signs of slowing down in their review of new medicines and proposed label expansions.
Outsourcing Pharma
JULY 26, 2024
Discover the groundbreaking work of Cure51, co-founded by Nicolas Wolikow and Simon Istolainen, revolutionizing cancer treatment through advanced AI and biotechnology.

Pharmacy Times
JULY 26, 2024
Study finds traces of “epigenetic scars” on the immune cells of patients who recovered from chronic hepatitis C infection.

Fierce Healthcare
JULY 26, 2024
Centene beat the Street even as Medicaid redeterminations pose a significant headwind heading into the back half of 2024, according to its
Pharmacy Times
JULY 26, 2024
The new approach could optimize precision medicine and lead to better outcomes across a variety of disease states.

The Checkup by Singlecare
JULY 26, 2024
The World Health Organization once predicted depression would be the leading cause of disease burden by 2030. Fortunately, antidepressants like Trintellix (vortioxetine) can help carry the weight of that burden. Like many drugs that treat major depressive disorder, Trintellix influences the brain’s serotonin levels to help regulate moods—but stopping treatment can get complicated.

Hospital Pharmacy Europe
JULY 26, 2024
The shingles vaccine that has been used in the NHS since last year is associated with a reduced risk of dementia, researchers have reported. A study of more than 200,000 adults before and after the US switched from Zostavax to the Shingrix vaccine found at least a 17% reduction in dementia diagnoses. The team from the University of Oxford also found a 23-27% lower risk of dementia in people who had been given Shingrix was compared to those who had not had Shingrix but had other vaccinations such

Outsourcing Pharma
JULY 26, 2024
In a candid interview at the DIA conference, James Bellamy, leader of the Solutions Consulting team at Qinecsa, shared his insights into the evolving landscape of pharmacovigilance (PV) technology.
Let's personalize your content