MHRA authorises new COVID-19 vaccine
European Pharmaceutical Review
AUGUST 2, 2023
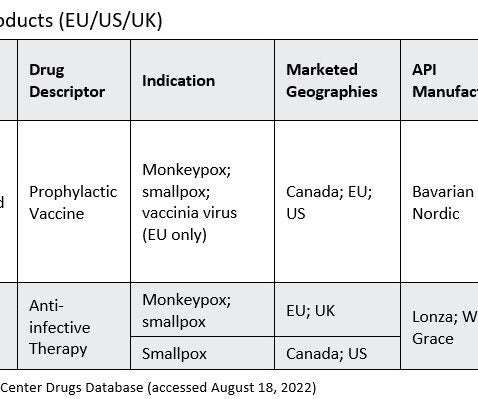
A new COVID-19 vaccine has been authorised by the Medicines and Healthcare products Regulatory Agency (MHRA). Bimervax is now the ninth vaccine to be authorised by the UK’s independent medicines regulator to treat the virus. This is an additional ingredient designed to trigger a stronger immune response.




























Let's personalize your content