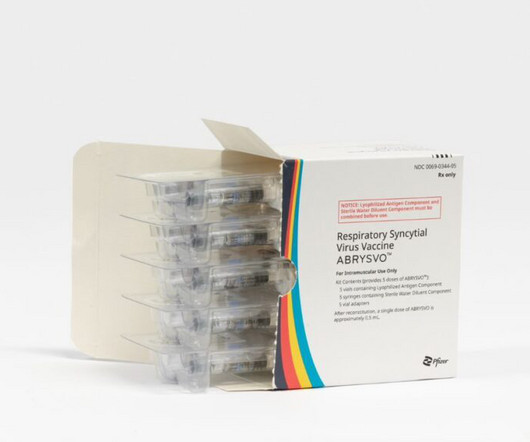
FDA approves Pfizer’s RSV vaccine designed to protect newborns by immunizing parent
STAT
AUGUST 21, 2023
The Food and Drug Administration on Monday approved a Pfizer vaccine that aims to protect newborns against RSV by vaccinating pregnant people in the latter part of pregnancy. The vaccine, Abrysvo, has also been approved for use in adults 60 and older to protect them against respiratory syncytial virus. Read the rest…




























Let's personalize your content