FDA advisory panel recommends a streamlined flu vaccine for next fall
STAT
MARCH 5, 2024
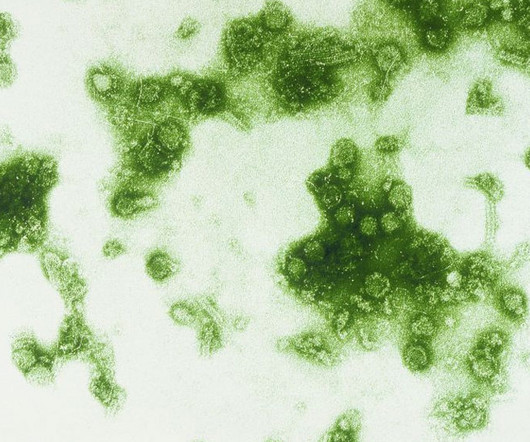
When Americans line up for flu vaccines next fall, they will almost certainly be getting vaccines that no longer contain protection against a family of flu viruses that appears to be extinct.






























Let's personalize your content