Recent Vaccine Approvals Present New Potential Errors
Prefilled diluent and vaccine syringes may be easily mixed up
Vaccines are one of the greatest success stories in public health, helping to eradicate or reduce many infectious diseases. However, if errors happen when prescribing, dispensing, or administering vaccines, the adverse impact on disease prevention could be significant. The success of any individual vaccine relies on its correct preparation and administration.
Image credit: Alernon77 | stock.adobe.com
We have previously written about preparation and administration errors with vaccines that require reconstitution (eg, the measles, mumps, and rubella vaccine [M-M-R II]) and 2 component vaccines (eg, the zoster vaccine and the diphtheria and tetanus toxoids, acellular pertussis, poliovirus, and Haemophilus b conjugate vaccine). In these cases, the wrong diluent was used to reconstitute the lyophilized powder component or the liquid component alone was administered to the patient. The potential for similar errors exists with recent product approvals that include syringes prefilled with diluent.
MERCK’S NEW PREFILLED DILUENT SYRINGES
Merck recently received FDA approval for new prefilled sterile diluent syringes and has begun distributing them (packaged separately) (Figure 1) for reconstituting a lyophilized powder vial of M-M-R II, varicella (Varivax), and the measles, mumps, rubella, and varicella (ProQuad) live virus vaccines. The diluent was formerly available only in vials.
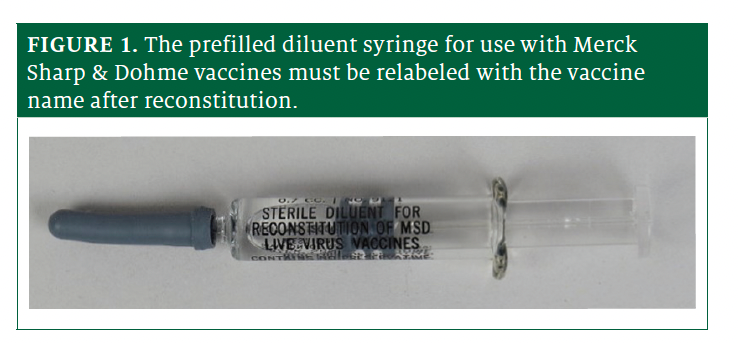
In general, prefilled syringes are useful and convenient. In this case, however, they are causing medication errors and creating increased risk. The syringes are labeled “Sterile diluent for reconstitution of [Merck Sharp & Dohme] live virus vaccines.” The company’s instructions and promotional materials say the syringes should be used to reconstitute the associated vaccine, withdraw the liquid back into the syringe, and then administer.1 However, the instructions do not mention the need to relabel the diluent syringe with the vaccine name after reconstitution. Therefore, if there are other prefilled syringes nearby (eg, on a table or countertop), all syringes will be labeled as sterile diluent in the same way and all look alike. That could lead to someone inadvertently picking up and injecting an unmixed diluent syringe or not knowing a syringe is already reconstituted, resulting in a patient receiving a double dose or 2 different vaccine products. Additionally, this removes the option of having parents read the syringe label as part of a process to confirm the right vaccine is about to be administered.
The Institute for Safe Medication Practices (ISMP) has already received an error report as well as a complaint about the situation. There are other vaccines such as GSK’s measles, mumps, and rubella vaccine (Priorix) and Pfizer’s respiratory syncytial virus (RSV) vaccine (Abrysvo) that use prefilled sterile diluent syringes and share the same problem.
INFLUENZA VACCINE MISTAKEN AS DILUENT MEANT FOR RSV VACCINE
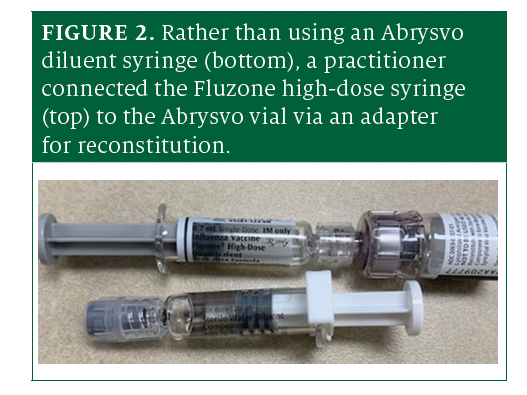
Pharmacies have reported 2 recent close calls involving patients who were supposed to concurrently receive influenza high-dose vaccine (Fluzone; Sanofi) and Abrysvo. Abrysvo is available in a vial containing lyophilized powder antigen. A practitioner must first dilute it using an accompanying prefilled syringe of sterile water for injection and a vial adapter. However, instead of connecting the Abrysvo diluent syringe to the vial, those preparing the vaccines mistakenly used Fluzone high-dose syringes (Figure 2). Fortunately, in both cases, the error was recognized prior to administration. The diluent syringe for Abrysvo looks similar to the Fluzone high-dose syringe, and the dark plunger stoppers make it difficult to read the labels. In addition, both the diluent syringe and the Fluzone syringe have Luer connectors, making them compatible with the vial adapter. A similar error could occur with other powdered vaccines when diluents and other vaccines are kept nearby and are in prefilled Luer-lock syringes.
SAFE PRACTICE RECOMMENDATIONS
Consider the following recommendations to prevent preparation and administration errors with vaccines that come with prefilled diluent syringes:
- Establish a process to keep vaccines and their corresponding diluents together if storage requirements do not differ.
- Prepare only 1 vaccine at a time. If patients require multiple vaccines and 1 vaccine requires reconstitution whereas others do not, start with the vaccine that needs reconstitution. After preparing and labeling the syringe, retrieve and ready the other vaccine.
- Implement barcode scanning prior to preparing and administering a vaccine. Configure the system to require scanning of both the vaccine and corresponding diluent barcodes.
- The ISMP has reached out to the FDA and manufacturers to notify them of these issues and recommended that manufacturers provide self-adhering labels, packaged with the specific vaccine, for use on the diluent syringe after the vaccine is reconstituted and withdrawn from the vial. For now, to reduce risk with these syringes, create vaccine-specific auxiliary labels to facilitate relabeling. Store the labels with the specific vaccine products.
About the Author
Michael J. Gaunt, PharmD, is a senior director of error reporting programs and editor at the Institute for Safe Medication Practices (ISMP) in Horsham, Pennsylvania. He also serves as the editor of the monthly ISMP Medication Safety Alert! Community/Ambulatory Care newsletter.
Reference
- One less step for prep: M-M-RII, VARIVAX, and ProQuad come with a prefilled diluent syringe. Merck. 2023. Accessed November 14, 2023. https://www.ismp.org/sites/default/files/attachments/2023-10/US-MMR-00348_MMRII-VARIVAX-Pro-Quad_PFS-Diluent-Downloadable-PDF_v9%20%28002%29.pdf

