Biosimilar Aflibercept Shows Positive Efficacy and Safety Results in Confirmatory Study
Pharmacy Times
AUGUST 17, 2023
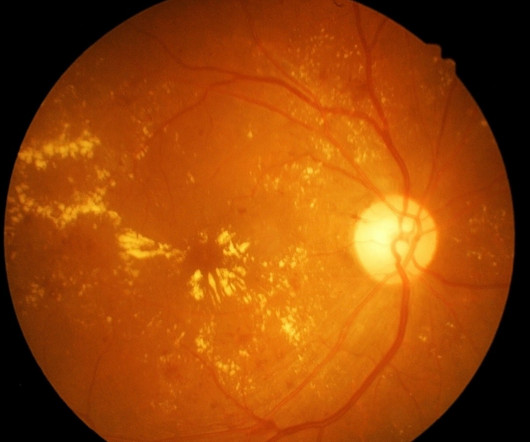
The Mylight phase 3 trial confirmed that there was no clinically meaningful differences between aflibercept and its reference biologic, Eylea, for patients with wet macular degeneration.






































Let's personalize your content