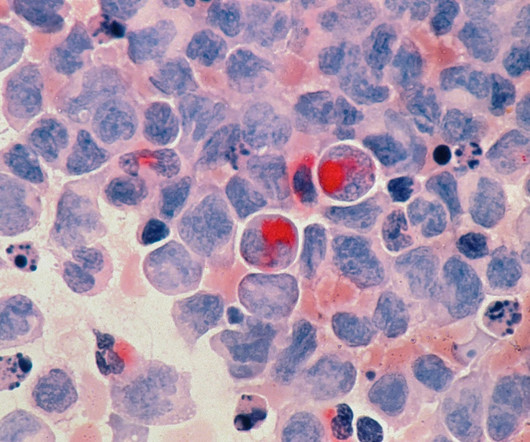
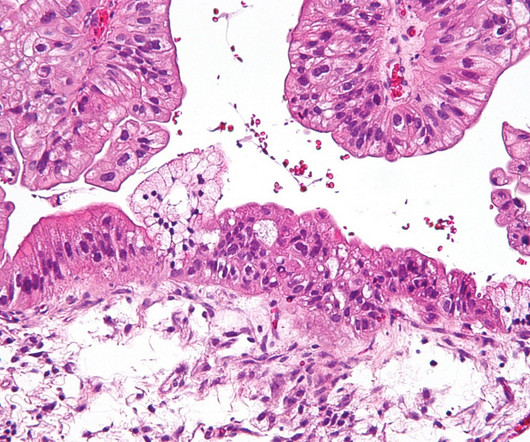
Mersana Therapeutics Reports US FDA’s Partial Clinical Hold on New Patient Enrollment of UpRi for Platinum-Sensitive Ovarian Cancer
PharmaShots
JUNE 16, 2023
Patients already enrolled can continue treatment The patient enrollment has been completed in the (UPLIFT) trial of UpRi for Pt-resistant ovarian cancer with results expected by early August.





































Let's personalize your content