FDA Approves Liso-Cel Therapy for Treatment of Adult Patients With CLL, SLL
Pharmacy Times
MARCH 15, 2024
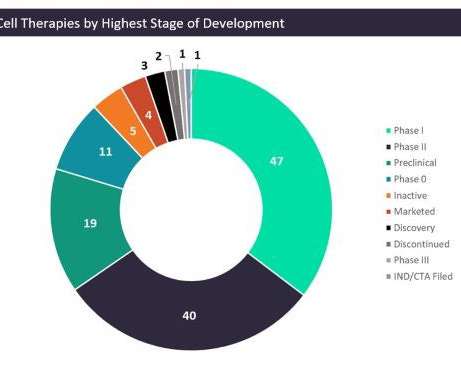
In a phase 1/2 clinical trial, lisocabtagene maraleucel helped patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) achieve complete response rates.





































Let's personalize your content