Comparison Of Rebyota Versus Vowst: A Study Table To Help You Compare Fecal Microbiota Therapies
IDStewardship
APRIL 14, 2024
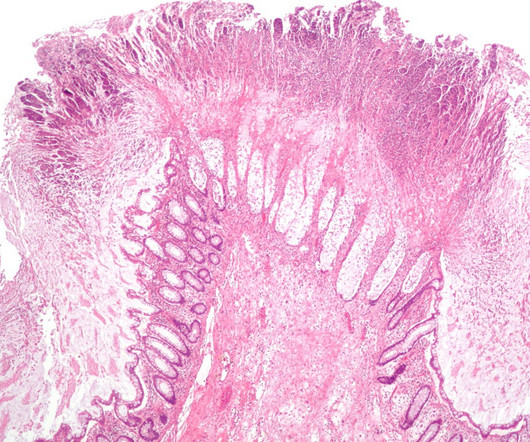
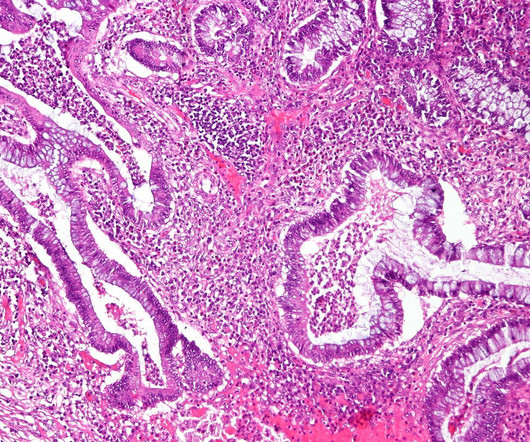
PharmD, BCPS, BCIDP, AAHIV-M Disclaimer : This text and table is intended for use as a study tool to assist people learning pharmacotherapy of recurrent Clostridioides difficile infections (rCDI). Article Posted 14 April 2024 What is Clostridioides difficile ? Clostridioides difficile ( C. Clostridioides difficile ( C.


































Let's personalize your content