L and J-HZ by L&J Bio for Herpes Zoster (Shingles): Likelihood of Approval
Pharmaceutical Technology
FEBRUARY 19, 2024
L and J-HZ is under clinical development by L&J Bio and currently in Phase I for Herpes Zoster (Shingles).

 clinical zoster
clinical zoster 
Pharmaceutical Technology
FEBRUARY 19, 2024
L and J-HZ is under clinical development by L&J Bio and currently in Phase I for Herpes Zoster (Shingles).
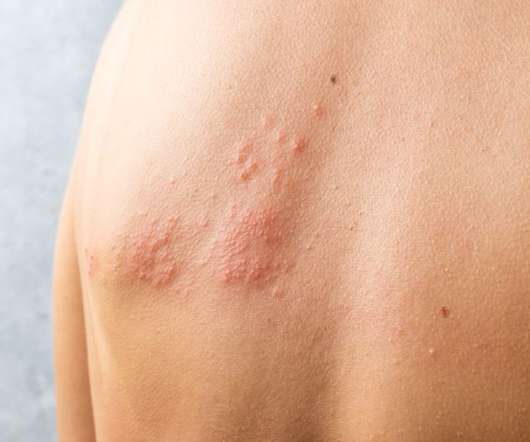
European Pharmaceutical Review
OCTOBER 21, 2022
GSK announced Shingrix (Zoster Vaccine Recombinant, Adjuvanted), the first approved shingles vaccine to combine a non-live antigen with a GSK-made adjuvant, can prevent shingles (herpes zoster) for at least decade. Shingles occurs when the varicella zoster virus (VZV) is reactivated in a patient.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
DECEMBER 20, 2023
VTP-400 is under clinical development by Barinthus Biotherapeutics and currently in Phase I for Herpes Zoster (Shingles).

The People's Pharmacy
JUNE 1, 2023
During seven years of follow-up, people who had received the herpes zoster vaccine were 20 percent less likely to develop dementia. They go on to add: “Our rigorous causal approach allows for the conclusion that herpes zoster vaccination is very likely an effective means of preventing or delaying the onset of dementia.
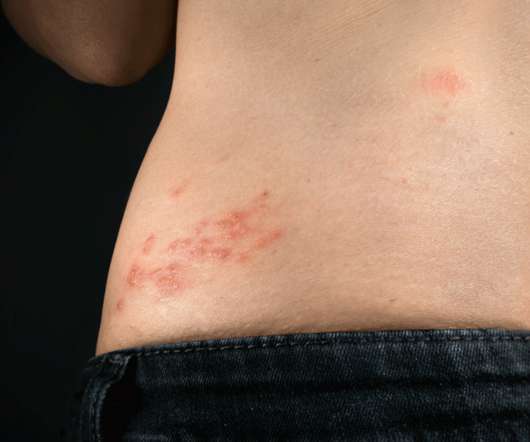
Hospital Pharmacy Europe
JANUARY 4, 2023
Infection with herpes zoster appears associated with a longer term higher risk of developing both a stroke and coronary heart disease. Herpes zoster infection and cardiovascular events. Herpes Zoster and Long-Term Risk of Cardiovascular Disease. Citation Curhan SG et al. J Am Heart Assoc.

Pharmaceutical Technology
JUNE 29, 2022
The Japanese Ministry of Health, Labour and Welfare (MHLW) has accepted GlaxoSmithKline’s (GSK) regulatory application for recombinant, adjuvanted Zoster vaccine, Shingrix, for preventing shingles (herpes zoster) in at-risk adults of the age 18 years and above.
pharmaphorum
JANUARY 5, 2022
Having already brought their mRNA-based CVID-19 vaccine Comirnaty to market at breakneck speed, Pfizer and BioNTech are hoping to fast-track a vaccine for shingles based on the same technology platform through clinical development, with trials due to start later this year.
Let's personalize your content