Clinical Overview: Tildrakizumab-asmn (Ilumya) for Plaque Psoriasis
Pharmacy Times
JULY 18, 2022
Tildrakizumab-asmn is a humanized IgG1/k monoclonal anti-IL-23 antibody indicated for the treatment of adults with moderate-to-severe plaque psoriasis.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

 clinical psoriasis
clinical psoriasis 
Pharmacy Times
JULY 18, 2022
Tildrakizumab-asmn is a humanized IgG1/k monoclonal anti-IL-23 antibody indicated for the treatment of adults with moderate-to-severe plaque psoriasis.

The Checkup by Singlecare
FEBRUARY 15, 2024
Psoriasis is an inflammatory condition affecting millions of Americans. The symptoms of psoriasis can overlap with other skin disorders such as eczema , and skin types, like dry skin. However, psoriasis isn’t just a skin condition—it’s an autoimmune disease.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
European Pharmaceutical Review
MARCH 13, 2024
The first and only investigational targeted oral peptide has been shown to maintain high rates of skin clearance in adults with moderate-to-severe plaque psoriasis through 52 weeks, new data shows. IL-23 has a key role in pathogenic T-cell activation in moderate-to-severe plaque psoriasis, the company added. at 16 weeks and 76.2
The Checkup by Singlecare
APRIL 16, 2024
Psoriasis is a skin condition that causes irritation, redness, and silvery scales. Most individuals with psoriasis have plaque psoriasis , which is when the silvery scales appear in patches. Up to 42% of patients with psoriasis also have psoriatic arthritis , a type of arthritis that affects people with psoriasis.

Hospital Pharmacy Europe
JUNE 22, 2023
The use of biologics in women with psoriasis who are either pregnant or planning to conceive is not associated with an increased risk of miscarriage, abortion or congenital malformations, according to the findings of a recent study. The team also included studies where women were planning to conceive and who were exposed to biologics.
European Pharmaceutical Review
JANUARY 24, 2024
Topline data from a novel Phase III study show that the biologic TREMFYA ® (guselkumab) facilitated rapid and significant clearance in moderate to severe scalp psoriasis ( PsO ) and significant improvement in scalp itch at 16 weeks, according to Johnson & Johnson. percent compared to 37.8 percent versus 33.4 percent compared to 37.8
Outsourcing Pharma
FEBRUARY 15, 2024
Artax Biopharma, a clinical-stage biotech company focused on transforming the treatment of autoimmune diseases, has dosed the first patient in its phase 2a trial evaluating oral, small molecule, AX-158 for the treatment of psoriasis.
STAT
NOVEMBER 6, 2023
SAN DIEGO — Ventyx Biosciences reported on Monday that it was ending development of an experimental drug for treating plaque psoriasis though it proved safe and moderately effective in a mid-stage trial. Within hours, the company’s stock tanked by more than 80%. Shares in Ventyx plummeted from $14.09 during after-hours trading.

STAT
DECEMBER 11, 2023
During 2022, drugmakers substantially raised prices on eight widely used medicines without any new clinical evidence to justify the increases, leading patients and health insurers in the U.S. to spend an additional $1.2 billion last year, according to a new report.
Drug Topics
JANUARY 20, 2023
Highly effective medications for severe disease are expensive and administered invasively, while the development of treatments for milder disease has lagged, a new review on psoriasis clinical trials concludes.

European Pharmaceutical Review
JANUARY 11, 2024
The biologic targets the p40 protein, which has key roles in treating immune-mediated diseases like Crohn’s disease, psoriasis as well as psoriatic arthritis, Alvotech highlighted. Uzpruvo is produced in Sp2/0 cells via perfusion, in the same way that the reference product Stelara is.

Pharmaceutical Technology
MARCH 18, 2024
Adalimumab biosimilar is under clinical development by Biocon and currently in Phase III for Plaque Psoriasis (Psoriasis Vulgaris).
European Pharmaceutical Review
MARCH 28, 2023
The European Commission (EC) has approved Sotyktu (deucravacitinib), a first-in-class, allosteric tyrosine kinase 2 (TYK2) inhibitor for adults with moderate-to-severe plaque psoriasis. Data that supported the approval EC’s approval was based on results from Bristol Myers Squibb’s Phase III POETYK PSO-1 and POETYK PSO-2 clinical trials.
Outsourcing Pharma
FEBRUARY 15, 2024
Artax Biopharma, a clinical-stage biotech company focused on transforming the treatment of autoimmune diseases, has dosed the first patient in its phase 2a trial evaluating oral, small molecule, AX-158 for the treatment of psoriasis.

Fierce Pharma
NOVEMBER 14, 2023
In the market, however, Vtama is struggling to catch on. As a result, Leerink Partners has slashed its sales projections for the nonsteroidal topical treatment.

Pharmacy Times
NOVEMBER 10, 2023
The sBLA was based on data from a clinical study assessing the pharmacokinetics of switching between adalimumab and adalimumab-bwwd for patients with moderate to severe plaque psoriasis.
Hospital Pharmacy Europe
APRIL 3, 2023
Psoriasis represents a chronic immune-mediated inflammatory skin disease with lesions characterised by hyper-proliferation of epidermal keratinocytes associated with inflammatory cellular infiltrate in both dermis and epidermis. Oral vitamin D supplementation and psoriasis disease severity A total of 122 participants with a mean age of 53.6
Hospital Pharmacy Europe
JUNE 19, 2023
To date, there are two further PDE inhibitors available: apremilast, which is an established oral therapy for psoriasis, and crisaborole, a topical agent used to treat atopic eczema. Psoriasis management Topical roflumilast 0.3% has gained gained FDA approval for use in patients with plaque psoriasis.

The Checkup by Singlecare
FEBRUARY 20, 2024
The need for better psoriasis treatments has been long-standing for years. Now, options for effective psoriasis relief have multiplied. Due to these recent developments, 8 million Americans and 30 million people worldwide with psoriasis have reason for optimism. It, too, got approval from the FDA in 2022.

pharmaphorum
FEBRUARY 1, 2021
It’s well recognised that people with psoriasis can develop depression or anxiety, particularly in moderate or severe cases where a sizeable part of the body can have skin lesions. It’s not the first time that Almirall has partnered with a digital health specialist to develop tools that complement its drug therapies for psoriasis.

European Pharmaceutical Review
DECEMBER 14, 2022
a wholly-owned subsidiary of Nimbus Therapeutics and Nimbus Therapeutics’ tyrosine kinase 2 (TYK2) inhibitor NDI-034858, an oral, potential best-in-class selective allosteric TYK2 inhibitor for multiple autoimmune diseases following successful recent Phase IIb results in psoriasis. For $4 billion , Takeda will acquire Nimbus Lakshmi, Inc.,

Pharmaceutical Technology
DECEMBER 20, 2023
Zasocitinib is under clinical development by Takeda Pharmaceutical and currently in Phase III for Plaque Psoriasis (Psoriasis Vulgaris).
European Pharmaceutical Review
JULY 4, 2023
JNJ-2113, the first and only oral interleukin-23 receptor (IL-23R) antagonist peptide in development for moderate-to-severe plaque psoriasis (PsO) has demonstrated positive results in a Phase II trial. How does Janssen’s oral IL-23R antagonist for plaque psoriasis work? at the World Congress of Dermatology 2023. .

Pharmaceutical Technology
DECEMBER 5, 2023
ONO-4685 is under clinical development by Ono Pharmaceutical and currently in Phase I for Plaque Psoriasis (Psoriasis Vulgaris).
Outsourcing Pharma
MARCH 24, 2022
The pharmaceutical company's clinical study is intended to examine critical gaps in care for people of color living with moderate to severe plaque psoriasis.

The Checkup by Singlecare
FEBRUARY 27, 2024
For centuries, oatmeal has been used as a treatment for skin irritations like rashes, burns, insect bites, eczema, and psoriasis. How to make an oatmeal bath Dermatology nurse practitioner Sherry Sanvictores frequently recommends a colloidal oatmeal bath to her patients suffering from inflammatory skin conditions like eczema and psoriasis.
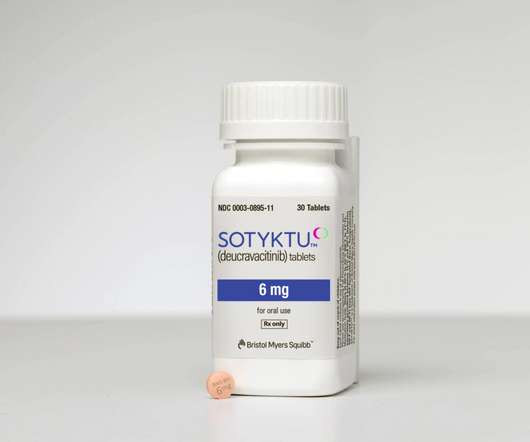
Pharmaceutical Technology
SEPTEMBER 12, 2022
The US Food and Drug Administration (FDA) has granted approval for Bristol Myers Squibb ’s (BMS) Sotyktu (deucravacitinib) to treat adult patients with moderate-to-severe plaque psoriasis. The approval is based on data from the Phase III POETYK PSO-1 and POETYK PSO-2 clinical trials.

The Checkup by Singlecare
NOVEMBER 17, 2023
Psoriasis Psoriasis is a long-term skin condition that causes scaly, itchy plaques on the skin. have psoriasis, according to the National Psoriasis Foundation. RELATED: Psoriasis vs. eczema vs. dry skin: How to tell the difference Stress and anxiety Sometimes, emotional distress can manifest physically.
pharmaphorum
OCTOBER 26, 2022
Psoriasis is a tricky disease to manage, as drawbacks and side effects loom with each and every type of treatment. Anyone who watches television has heard the list of potential adverse reactions, considering that commercials for psoriasis treatments are seemingly only outnumbered by goofy ads for auto insurance. Manage it early.

STAT
MARCH 18, 2023
In an intermediate-stage clinical study of 259 people with moderate-to-severe plaque psoriasis, the highest dose of the daily pill completely cleared itchy and painful patches of skin in one-third of the patients after 12 weeks.

Outsourcing Pharma
MAY 26, 2023
The first patients with psoriasis have been dosed with a new drug to help combat the inflammatory skin disease in a clinical trial in China.

Pharmafile
APRIL 12, 2023
ALM) , a global biopharmaceutical company focused on skin health, today announced the publication in the British Medical Journal (BMJ) of the POSITIVE study protocol, the first clinical study in dermatology to assess patients’ wellbeing as a primary endpoint. April 12, 2023 – Almirall, S.A. read more
European Pharmaceutical Review
JUNE 29, 2023
This is relevant in Crohn’s disease, ulcerative colitis and chronic skin disease psoriasis. Previous research has shown that blocking interleukin-23 with specific therapeutic antibodies is very effective in psoriasis and Crohn’s disease. Mirikizumab is the first such antibody tested for ulcerative colitis. percent versus 13.3

STAT
AUGUST 7, 2023
” As a result, Lilly “botched” the analysis of early clinical trial data in which Rezpeg was tested as a treatment for eczema and psoriasis. Continue to STAT+ to read the full story…

PharmaShots
JANUARY 23, 2023
The specific mechanisms by which VTAMA cream exerts its therapeutic action in psoriasis patients are unknown. Until recently, there were very few topical options available for treating plaque psoriasis long-term. Smriti: Brief us about the study design of the P-III clinical trial evaluating VTAMA cream. of VTAMA cream?
Pharma Mirror
SEPTEMBER 16, 2022
Barcelon, a global biopharmaceutical company focused on skin health, announced at the 31st EADV (European Association of Dermatology and Venereology) Congress the results from TRIBUTE, an interventional phase IV clinical study, which resembled real-life clinical practice. In this study, Ilumetri®?

pharmaphorum
DECEMBER 22, 2021
Amgen has won FDA approval for a stronger label for its oral plaque psoriasis therapy Otezla, as it prepares for competition from Bristol-Myers Squibb’s much-touted rival deucravacitinib, which could make its debut next year. The post Amgen builds Otezla’s psoriasis label as rival BMS looms large appeared first on.

PharmaShots
JUNE 22, 2023
in patients with HS The results showed a clinical improvement for patients who completed the planned 16wks. The results were consistent with the results from the P-IIb dose-finding study (IASOS) in psoriasis The results will be presented at an upcoming scientific conference. of treatment, improvements incl.

Pharmaceutical Technology
JULY 12, 2022
Health Canada has accepted to review Arcutis Biotherapeutics’ New Drug Submission (NDS) for roflumilast cream 0.3% (ARQ-151) to treat plaque psoriasis in adult and adolescent patients. The NDS submission by Arcutis is based on positive findings from the company’s pivotal Phase III programme and two long-term open-label clinical trials.
Hospital Pharmacy Europe
APRIL 23, 2024
It also marks the fourth marketing authorisation for the drug, adding to the existing indications in moderate-to-severe plaque psoriasis, active psoriatic arthritis and active axial spondyloarthritis. This approval makes bimekizumab the first biologic for moderate-to-severe HS to target IL-17F in addition to IL-17A.

The Checkup by Singlecare
NOVEMBER 6, 2023
Those on immunosuppressive drugs for psoriasis or ulcerative colitis may soon breathe a little easier when they get to the pharmacy counter: The U.S. Manufactured by Amgen, the biosimilar—which is like a generic for biologic medications—was approved to treat inflammatory conditions like plaque psoriasis or ulcerative colitis.

PharmaShots
APRIL 17, 2023
Daniel Quirk: Psoriasis is a widely prevalent, chronic, systemic immune-mediated disease that affects approximately 7.5 Daniel Quirk: Psoriasis is a widely prevalent, chronic, systemic immune-mediated disease that affects approximately 7.5 million people in the United States. million people in the United States.

pharmaphorum
JULY 19, 2021
During the last two decades, the landscape for psoriasis treatment has exploded. Suddenly, those with more severe psoriasis had safer and more effective treatment options that delivered the life-changing potential for clear skin. National Psoriasis Foundation found 52% of patients with psoriasis are dissatisfied with treatment.
European Pharmaceutical Review
APRIL 26, 2024
Recent biosimilar developments In February 2024, the EMA’s Committee for Medicinal Products for Human Use (CHMP) granted a positive opinion for Pyzchiva as a biosimilar to treat the autoimmune condition plaque psoriasis.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content