Annex 1 in Force! Join the First Discussion after the 25 August Effective Date
ISPE
SEPTEMBER 21, 2023
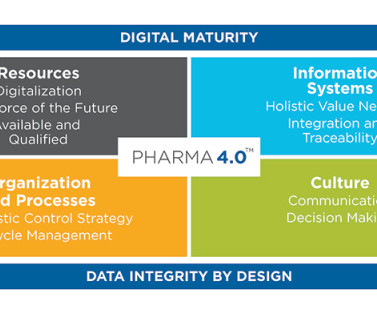
Gorton 21 September 2023 PIC/s Annex 1, and the WHO Annex 2 for the manufacturing of sterile products took effect on 25 August 2023, and the time to attend the 2023 ISPE Pharma 4.0™ As shown in the pictures, barrier systems such as RABS or Isolators, Material Airlock, E-Beam, and RTPs (Rapid Transfer Ports) are seen as technical solutions.















































Let's personalize your content