Aggregation in pharma: A must have for supply chain traceability
Pharmaceutical Commerce
APRIL 2, 2024
Business strategies and top news in the biotech / biopharma industry, including market access, supply chain distribution and more.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

 market-access news
market-access news 
Pharmaceutical Commerce
APRIL 2, 2024
Business strategies and top news in the biotech / biopharma industry, including market access, supply chain distribution and more.

Pharmaceutical Commerce
FEBRUARY 4, 2024
Business strategies and top news in the biotech / biopharma industry, including market access, supply chain distribution and more.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Commerce
JANUARY 23, 2024
Business strategies and top news in the biotech / biopharma industry, including market access, supply chain distribution and more.
European Pharmaceutical Review
MARCH 10, 2023
million Government grant and is designed to expand the UK’s ability to manufacture new gene and cell therapies. The CBC will help the UK grow its cell and gene therapy industry in a rapidly growing international market.” This inevitably delays clinical trials and patients’ access, and often increases costs.

DiversifyRx
OCTOBER 12, 2022
Let’s explore how you can use the DEA Drug Takeback day in October to get new patients to your pharmacy for FREE! When it comes to pharmacy marketing, I am all about marketing in many different ways and using more of a guerilla marketing method as a foundation before you start paying for a bunch of advertising.

PharmaShots
MAY 24, 2023
Alvotech will be responsible for the development and commercial supply Advanz Pharma will leverage its existing specialty and hospital capabilities in the EU to ensure successful market registration, commercialization, and patient access. Your go-to media platform for customized news ranging for multiple indications.

Pharmaceutical Technology
MAY 26, 2023
The European Medicines Agency (EMA) has accepted two marketing authorisation applications (MAA) from Sandoz for the proposed biosimilar denosumab. If approved, this has the potential to provide people living with osteoporosis and cancer of the bone or bone metastasis access to a cost-effective and high-quality treatment option.

PharmaShots
APRIL 4, 2023
Shots: The EC has granted marketing authorization for Hyrimoz (adalimumab-adaz) high-concentration formulation, a biosimilar to Humira (adalimumab) in the EU. Hyrimoz has been approved for use in all the indications covered by the reference Humira, incl.

PharmaShots
APRIL 26, 2023
Spectrum’s combined assets & commercial infrastructure will accelerate Rolvedon’s launch for patients' benefit & drive further growth The combination of Assertio’s Omni-Channel digital sales capabilities & Rolvedon's in-person commercial team will enhance market access & growth across all products Ref: Globenewswire (..)

PharmaShots
MAY 10, 2023
Your go-to media platform for customized news ranging for multiple indications. For more information connect with us at connect@pharmashots.com

pharmaphorum
AUGUST 21, 2020
New insights into smell and taste loss symptoms, artificial intelligence and further encouraging data from a vaccine candidate hit the headlines this week as the COVID-19 coronavirus pandemic continues. Here we highlight the biggest R&D, market access and digital coronavirus news of the past week.

PharmaShots
APRIL 11, 2023
Shots: The company received marketing authorization from Health Canada for expanded use of Orkambi to treat cystic fibrosis in children aged 1-<2yrs. The company will collaborate closely with payers to secure access for this new patient population shortly. P-III study evaluating Orkambi in 46 children aged 1 to <2yrs.

PharmaShots
APRIL 25, 2023
The product is now available to patients in 9 global markets, incl. the US for POMC, PCSK1, or LEPR deficiency obesity and/or BBS Ref: Rhythm | Image: Rhythm Related News:- Rhythm’s Imcivree (setmelanotide) Receives EC’s Approval for the Treatment of Bardet-Biedl Syndrome

European Pharmaceutical Review
OCTOBER 14, 2022
Amgen’s 2022 market report has detailed how competition has driven savings across healthcare, estimating that a considerable number of biologics will be in competition with biosimilars in five to 10 years. New biosimilars allow more companies to invest additional funds in novel, pioneering treatments.

European Pharmaceutical Review
SEPTEMBER 11, 2023
About the biosimilar development deal This agreement will help to expand access to biosimilar medicines , according to Samsung Bioepis. Adalimumab biosimilars shaping market, research states Last month, Sandoz announced it was going to seek regulatory approval for its aflibercept biosimilar in the US and EU in the coming months.
Pharmaceutical Technology
NOVEMBER 14, 2022
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has granted marketing authorisation for Oncopeptides’ Pepaxti (melphalan flufenamide; melflufen) along with dexamethasone to treat multiple myeloma patients. Currently, the company is analysing Pepaxti’s market access options in the country.

pharmaphorum
JANUARY 20, 2022
This news will likely be welcomed by sufferers, as, currently, there is no cure for UC. Galapagos, based in Belgium, is responsible for the commercialisation of the product in Europe, while Gilead holds the rights outside of Europe, including in Japan, where it is co-marketed with Eisai.

European Pharmaceutical Review
OCTOBER 28, 2022
A new report , Addressing Europe’s Medicine Exodus by Teva called on legislators to ensure a safer, more secure supply of essential medicines for EU patients, by cutting reliance on low-cost markets. Newer markets often offer high state subsidies, lower labour costs and energy prices at a fraction of Western Europe’s.
pharmaphorum
JANUARY 31, 2022
It is becoming increasingly important for people all over the world to understand the importance of vaccines and to have access to the vaccines they need – especially with digital innovation driving new vaccine development. Pfizer’s Josh Raysman, Saad Saeed, and Shanaya Deboo discuss vaccine awareness, access, and innovation.

Hospital Pharmacy Europe
OCTOBER 20, 2023
While current DLBCL treatments such as CAR T therapies are provided in specialist centres across England, glofitamab can be offered at more cancer treatment sites across the country, improving timely access. Glofitamab gained conditional marketing authorisation in the EU in July 2023.

European Pharmaceutical Review
AUGUST 26, 2022
Moderna pledged in October 2020 that, to enable equitable global access to vaccines, it would note enforce its COVID-19 related patents while the pandemic continued. As of March 2022, Moderna says that the situation has changed, and with vaccine supply no longer a barrier to access in many parts of the world, it is now updating the pledge.
Hospital Pharmacy Europe
OCTOBER 23, 2023
The injectable bispecific antibody epcoritamab (brand name Tepkinly) has been granted conditional marketing authorisation by the Medicines and Healthcare products Regulatory Agency (MHRA), its manufacturer Abbvie has announced. This follows its conditional marketing authorisation by the European Commission in late September 2023.
pharmaphorum
SEPTEMBER 30, 2022
” (1/2) BREAKING NEWS: @US_FDA approved AMX0035, a new treatment that has been shown to slow progression of #ALS and extend life. ” In both the US and Canada, staying on the market will depend on the outcome of the 600-patient PHOENIX study in two years. . Bad news for Biohaven. pic.twitter.com/Kck0tp1nSJ.

pharmaphorum
NOVEMBER 18, 2020
The decision means that with two CGRP antibodies now cleared for migraine prevention, the first drug in the class to be approved in Europe – Novartis’ Aimovig (erenumab) – has fallen further behind its rivals in getting access to the UK market.

pharmaphorum
NOVEMBER 18, 2020
The decision means that with two CGRP antibodies now cleared for migraine prevention, the first drug in the class to be approved in Europe – Novartis’ Aimovig (erenumab) – has fallen further behind its rivals in getting access to the UK market.

pharmaphorum
FEBRUARY 8, 2021
In the third part of this series taking stock of t he 2019 Voluntary Scheme for Branded Medicines Pricing and Access objectives, Leela Barham looks at the third VPAS objective that relates to the economy and innovation. That said, there is good news on the R&D front. Encouragement of R&D. But not by much; it went from £377.3

pharmaphorum
JULY 11, 2022
“Improving access to new therapies is crucial to enable clinicians to select the most appropriate therapy for each individual patient, so this is welcomed news for those living with PsA,” she said. The post NICE makes J&J’s Tremfya more accessible in England and Wales appeared first on.

The Checkup by Singlecare
NOVEMBER 18, 2022
Liletta (levonorgestrel-releasing intrauterine system) 52 mg was deemed safe for this extended-use time period on the basis of data from the ACCESS IUS (A Comprehensive Contraceptive Efficacy & Safety Study of an IUS) published earlier this year in the American Journal of Obstetrics & Gynecology. Potential for even longer usage.
STAT
NOVEMBER 18, 2022
The monoclonal antibody teplizumab, which will be marketed under the brand name Tzield and is from ProventionBio and Sanofi, is given through intravenous infusion. Diabetic patients will now have easier access to insulin after the FDA approved an Eli Lilly biosimilar as interchangeable with the biologic drug , Bloomberg News notes. Rezvoglar,

European Pharmaceutical Review
APRIL 11, 2024
The centre is helping to set out a vision for a more sustainable future for the sector, by working with businesses across the industry and giving them access to state-of-the-art facilities and academic knowledge. This allows a more detailed understanding of exactly how different variables affect the outcomes.

pharmaphorum
JUNE 9, 2021
Our ambitious growth strategy plays a crucial role in helping us to achieve our mission of improving access and empowering healthcare professionals and patients with the knowledge needed to save lives. The post Welcoming analytics-driven digital marketing agency closerlook to the Fishawack Health pack appeared first on.

pharmaphorum
FEBRUARY 24, 2022
Following a period of restricted access, the once-a-day capsule will now be recommended for routine use in the NHS. . Initially, NICE cleared niraparib to be made available through the Cancer Drugs Fund (CDF) – a programme designed to increase the speed of drugs to market within spending margins – in 2021.

pharmaphorum
APRIL 26, 2022
The news is a timely boost for Paris, France-based Valneva, which is facing uncertain prospects for its COVID-19 vaccine after the EU regulator requested more data on the latecomer candidate this week despite a UK approval earlier in April. The post Readout sets up phase 3 for Valneva/Pfizer’s Lyme disease jab appeared first on.
pharmaphorum
JANUARY 12, 2022
Biogen and partner Eisai had been hoping for good news from the review of Aduhelm (aducanumab) by the Centre for Medicare and Medicaid Services (CMS), but the draft decision means broad coverage of the drug will have to wait for the outcome of ongoing studies before it can hope for broader coverage.

pharmaphorum
JULY 20, 2022
It might not have been much in the way of news coverage, but it was picked up in a few places. That savings come from patents expiring is not news, but it does highlight how there can be a major impact on spend. Freeing up spend for fast access to innovative medicines. billion in three years. Genericisation. No mention of VPAS.

Fuld
APRIL 15, 2021
In just three years, Amazon has put together a package of leading-edge services that have the potential to revolutionize the US healthcare market. And, all of this is accessed through best-in-class online customer portals and supported by 24/7 online customer service. Healthcare Market. 2018 — Haven, PillPack, and Aiva.

pharmaphorum
MARCH 4, 2022
New survey data from Press Ganey suggests that pharma may be able to get an edge by integrating physician directories into brand websites. But in 2022, with digital communication ubiquitous, pharma companies are finding another option: market to consumers, then help them select a physician.
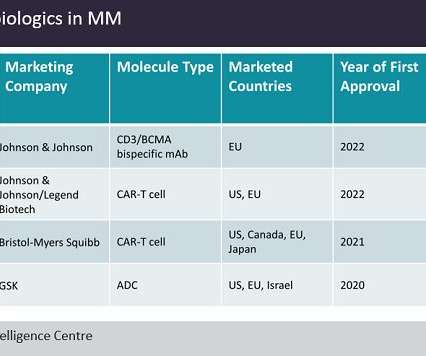
Pharmaceutical Technology
SEPTEMBER 2, 2022
Multiple myeloma (MM) therapeutics comprise a multi-billion dollar market, among the most valuable in oncology. Last month, the European Medicines Agency's (EMA) approval of Johnson & Johnson’s (J&J) anti-CD3/BCMA bispecific antibody Tecvayli (teclistamab) marked the fourth anti-BCMA biologic to enter the R/R MM market.

pharmaphorum
OCTOBER 12, 2021
The approval brought the drug into direct competition with Ongentys (opicapone) from Neurocrine Biosciences, which was approved for the same use last year, and having access to Supernus’ sales force will help in the marketing battle between the two drugs. per share subject to Gocovri meeting sales targets.

Roots Analysis
JANUARY 2, 2024
Patient engagement solutions include electronic medical records (HER), telemedicine and patient portals, which improve communication and help patients access their healthcare information. What are Applications of Patient Engagement? Detailed information on all suggestions to improvise the overall app is available in the full report.

pharmaphorum
AUGUST 7, 2020
Find out more in our roundup of the biggest R&D, market access and digital stories from the past week. The post Coronavirus pharma news roundup – 07/08/20 appeared first on. Pharma’s reputation has soared due to the COVID-19 pandemic – but coronavirus is starting to bite companies’ sales.

pharmaphorum
JULY 27, 2022
Despite the UK’s potential as an international life sciences leader, it lags many competitors in a number of key metrics, including access to new medicines and global share of clinical trial recruitment. Access to medicines. Latest UK Life Sciences Competitive Indicators “ought to ring alarm bells across Government,” says ABPI.

pharmaphorum
DECEMBER 10, 2020
The European Medicines Agency (EMA) says it suffered a cyberattack, with documents relating to a Pfizer and BioNTech’s COVID-19 vaccine accessed. Shortly after however BioNTech confirmed that documents submitted as part of its marketing application for coronavirus vaccine BNT-162b had been accessed by the hackers.
STAT
DECEMBER 5, 2022
… Johnson & Johnson said it does not intend to make an offer for Horizon Therapeutics, days after the developer of medicines for rare autoimmune and inflammatory diseases revealed it was in talks with the company , Bloomberg News writes. regulatory approval, Bloomberg News says. Food and Drug Administration.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content