Clinicians Use SSRIs Off- Label in Many Conditions
As the body of data has grown, clinicians’ propensity to use selective serotonin reuptake inhibitors off-label has increased
When selective serotonin reuptake inhibitors (SSRIs) were first introduced, researchers hailed them as the first rationally designed psychotropic drug because they were developed with a specific target in mind: selective inhibition of 5-hydroxytryptamine reuptake.1 Since the FDA approved fluoxetine (Prozac; Eli Lilly) in 1987, SSRIs have dominated the treatment of depression in the United States.2
Image credit: Natallia | stock.adobe.com
FDA approved for bulimia nervosa, generalized anxiety disorder, major depressive disorder, obsessive-compulsive disorder, panic disorder, and posttraumatic stress disorder, SSRIs are dispensed often in pharmacies. Three SSRIs (fluoxetine, paroxetine, and sertraline) are FDA approved for premenstrual syndrome and premenstrual dysphoric disorder.2-4
SSRIs selectively block serotonin (5-hydroxytryptamine) transporters, increasing their levels at the neuron’s somatodendritic and presynaptic ends.5 Targeting this site reduced the number of adverse effects and improved the safety profile over other classes of antidepressants in clinical trials.6,7 As the data about SSRIs’ safety and efficacy profile accumulated, so, too, did the propensity for clinicians to use these medications off-label.
BODY DYSMORPHIC DISORDER (BDD)
BDD manifests as debilitating obsessions on single or multiple body parts (eg, ears, face, hair, nose) and is often associated with compulsive counter behaviors that affect quality of life.8,9 Because the SSRIs address obsessive pathology, researchers have proposed they could be effective in BDD. Although serotoninergic signaling seems to be somewhat impaired in BDD, only a few small studies demonstrate SSRIs’ utility. Four open-label studies found that escitalopram (Lexapro; AbbVie), citalopram (Celexa; Allergan), and fluvoxamine (Luvox; Jazz Pharmaceuticals) improved BDD in more than half of participants when given with cognitive behavioral therapy (CBT).10-13
IMPULSE CONTROL DISORDER (ICD)
Impulsivity (the tendency to act abruptly without reflection or forethought) often manifests as kleptomania, pyromania, intermittent explosive disorder, and pathological gambling.9 Serotonin might modulate impulsive behaviors in the prefrontal cortex.14 The most common SSRI employed in ICD is fluoxetine 20 mg/d to 60 mg/d, but some clinicians have used citalopram 20 mg/d to 60 mg/d. Large, well-structured studies addressing specific types of impulsivity are lacking, and more work is needed to establish efficacy.15
IRRITABLE BOWEL SYNDROME (IBS)
IBS—a functional disorder that includes diarrhea, constipation, bloating, and abdominal pain—emanates from multiple etiologies, but psychological factors contribute.16 When antidepressants are used, tricyclic antidepressants have proven the most effective of the antidepressants, but some results with SSRIs have been encouraging. A review and metaanalysis including trials of SSRIs compared with placebo found that half of participants in active treatment groups reported significantly reduced IBS symptoms.17 Clinical trials employing paroxetine 5 mg/d to 20 mg/d reported significant improvements in patient-reported gastrointestinal symptoms and clinical evaluations.18
MIGRAINE
Theoretically, reduced serotonin levels may be instrumental in migraine’s pathogenesis, as researchers have identified low serotonin levels and brief upregulation during attacks.19,20 Typical off-label SSRI use in migraine is for prophylaxis. Regardless, compared with other antidepressants like tricyclic antidepressants, SSRIs tend to be less effective, possibly because they do not cause sedation.21 Fluoxetine has been studied most at doses between 20 mg/d and 40 mg/d.21,22 The American Academy of Neurology and American Headache Society guidelines bestow a rating of U (inadequate or conflicting data) for fluoxetine and fluvoxamine in migraine.23 One consideration of concern is that headache is a common adverse effect associated with SSRIs.24
PARAPHILIAS AND HYPERSEXUALITY
Some clinicians have used SSRIs for paraphilic disorders (recurrent, intense, sexually arousing fantasies, sexual urges, or behaviors) and hypersexuality (compulsive sexual behavior). Evidence suggests enhancing serotonin levels can suppress sexual drive, and SSRIs may address the compulsive element of these diagnoses.25,26 For these reasons, the World Federation of Societies of Biological Psychiatry recommends trying SSRIs in mild paraphilic disorders with obsessive features unresponsive to CBT.25 However, few studies are available to provide firm evidence of SSRIs’ benefit.
PREMATURE EJACULATION
Up to 40% of men experience premature ejaculation. Serotonin contributes to regulation of ejaculatory reflex, and SSRIs may delay ejaculation. A meta-analysis evaluating long-acting and short-acting SSRIs found that paroxetine was associated with the best results.28 Some prescribers use once-daily, chronic dosing, but on-demand regimens have also been used.2
CONCLUSION
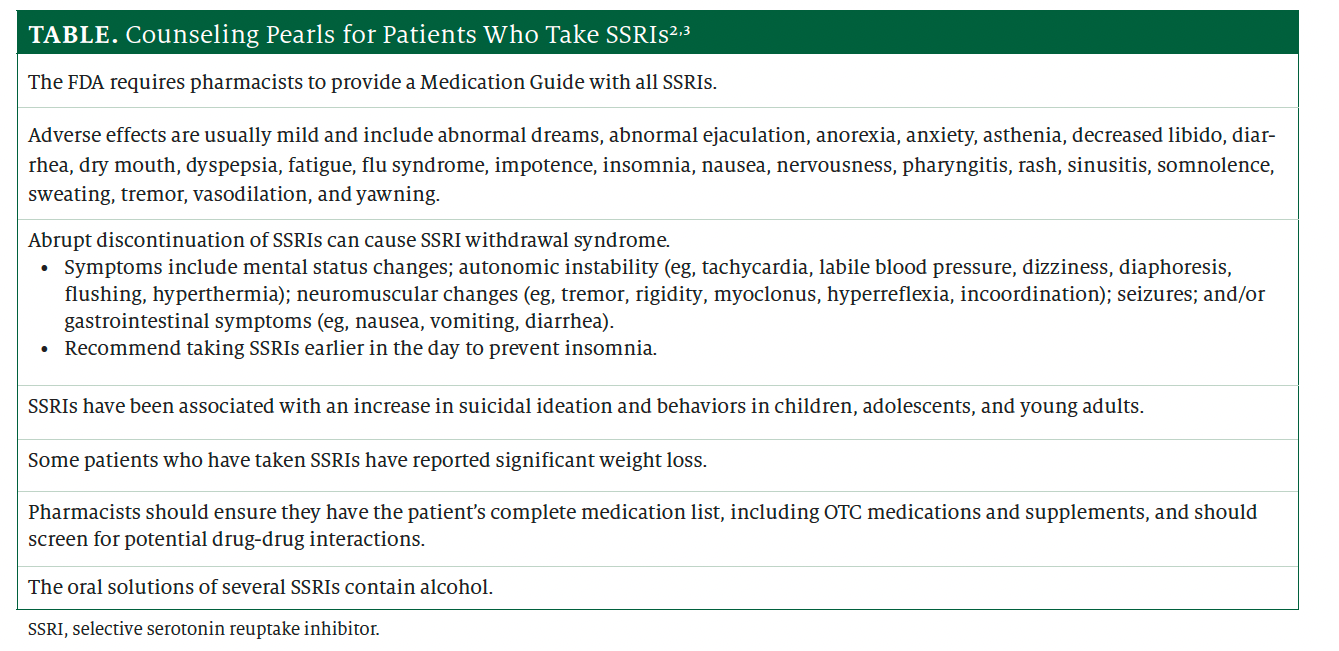
Whenever patients take SSRIs, counseling is in order. The Table2,3 lists key counseling points. When patients use SSRIs for off-label indications, they may have questions or concerns. Pharmacists are well positioned to help address these concerns and guide both patients and health care professionals in selecting the most appropriate treatment regimen.
About the Author
Jeannette Y. Wick, RPH, MBA, FASCP, is the director of the Office of Pharmacy Professional Development at the University of Connecticut School of Pharmacy in Storrs.
References
- Robinson E. Psychopharmacology: from serendipitous discoveries to rationale design, but what next? Brain Neurosci Adv. 2018;2:2398212818812629. doi:10.1177/2398212818812629
- Prozac. Prescribing information. Eli Lilly and Company; 2023. Accessed November 12, 2023. https://pi.lilly.com/us/prozac.pdf
- Zoloft. Prescribing information. Pfizer; 2021. Accessed November 12, 2023. https://labeling.pfizer.com/ShowLabeling.aspx?id=517
- Paxil. Prescribing information. GlaxoSmithKline; 2021. Accessed November 12, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
- Faquih AE, Memon RI, Hafeez H, Zeshan M, Naveed S. A review of novel antidepressants: a guide for clinicians. Cureus. 2019;11(3):e4185. doi:10.7759/cureus.4185
- Qin B, Zhang Y, Zhou X, et al. Selective serotonin reuptake inhibitors versus tricyclic antidepressants in young patients: a meta-analysis of efficacy and acceptability. Clin Ther. 2014;36(7):1087-1095. doi:10.1016/j.clinthera.2014.06.001
- Coupland C, Hill T, Morriss R, Moore M, Arthur A, Hippisley-Cox J. Antidepressant use and risk of adverse outcomes in people aged 20-64 years: cohort study using a primary care database. BMC Med. 2018;16(1):36. doi:10.1186/s12916-018-1022-x
- Mufaddel A, Osman OT, Almugaddam F, Jafferany M. A review of body dysmorphic disorder and its presentation in different clinical settings. Prim Care Companion CNS Disord. 2013;15(4):PCC.12r01464. doi:10.4088/PCC.12r01464
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association Publishing; 2013.
- Phillips KA. An open-label study of escitalopram in body dysmorphic disorder. Int Clin Psychopharmacol. 2006;21(3):177-179. doi:10.1097/01.yic.0000194378.65460.ef
- Perugi G, Giannotta D, Di Vaio S, Frare F, Saettoni M, Cassano GB. Fluvoxamine in the treatment of body dysmorphic disorder (dysmorphophobia). Int Clin Psychopharmacol. 1996;11(4):247-254. doi:10.1097/00004850-199612000-00006
- Phillips KA, Albertini RS, Rasmussen SA. A randomized placebo-controlled trial of fluoxetine in body dysmorphic disorder. Arch Gen Psychiatry. 2002;59(4):381-388. doi:10.1001/archpsyc.59.4.381
- Phillips KA, Dwight MM, McElroy SL. Efficacy and safety of fluvoxamine in body dysmorphic disorder. J Clin Psychiatry. 1998;59(4):165-171. doi:10.4088/jcp.v59n0404
- New AS, Buschsbaum MS, Hazlett EA, et al. Fluoxetine increases relative metabolic rate in prefrontal cortex in impulsive aggression. Psychopharmacology (Berl). 2004;176(3-4):451-458. doi:10.1007/s00213-004-1913-8
- Jannini TB, Lorenzo GD, Bianciardi E, et al. Off-label uses of selective serotonin reuptake inhibitors (SSRIs). Curr Neuropharmacol. 2022;20(4):693-712. doi:10.2174/1570159X19666210517150418
- Mayer EA. Clinical practice: irritable bowel syndrome. N Engl J Med. 2008;358(16):1692-1699. doi:10.1056/NEJMcp0801447
- Kułak-Bejda A, Bejda G, Waszkiewicz N. Antidepressants for irritable bowel syndrome-a systematic review. Pharmacol Rep. 2017;69(6):1366-1379. doi:10.1016/j.pharep.2017.05.014
- Stasi C, Caserta A, Nisita C, et al. The complex interplay between gastrointestinal and psychiatric symptoms in irritable bowel syndrome: a longitudinal assessment. J Gastroenterol Hepatol. 2019;34(4):713-719. doi:10.1111/jgh.14375
- Deen M, Christensen CE, Hougaard A, Hansen HD, Knudsen GM, Ashina M. Serotonergic mechanisms in the migraine brain - a systematic review. Cephalalgia. 2017;37(3):251-264. doi:10.1177/0333102416640501
- Altamura C, Corbelli I, de Tommaso M, et al. Pathophysiological bases of comorbidity in migraine. Front Hum Neurosci. 2021;15:640574. doi:10.3389/fnhum.2021.640574
- Burch R. Antidepressants for preventive treatment of migraine. Curr Treat Options Neurol. 2019;21(4):18. doi:10.1007/s11940-019-0557-2
- Saper JR, Silberstein SD, Lake AE 3rd, Winters ME. Fluoxetine and migraine: comparison of double-blind trials. Headache. 1995;35(4):233. doi:10.1111/j.1526-4610.1995.hed3504233_1.x
- Tfelt-Hansen PC. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2013;80(9):869-870. doi:10.1212/01.wnl.0000427909.23467.39
- Alemdar M, Selekler HM. Migraine with aura triggered by citalopram. Neuropsychobiology. 2007;55(2):121-122. doi:10.1159/000104469
- Thibaut F, Cosyns P, Fedoroff JP, et al. The World Federation of Societies of Biological Psychiatry (WFSBP) 2020 guidelines for the pharmacological treatment of paraphilic disorders. World J Biol Psychiatry. 2020;21(6):412-490. doi:10.1080/15622975.2020.1744723
- Turner D, Schöttle D, Bradford J, Briken P. Assessment methods and management of hypersexuality and paraphilic disorders. Curr Opin Psychiatry. 2014;27(6):413-422. doi:10.1097/YCO.0000000000000099
- Kalejaiye O, Almekaty K, Blecher G, Minhas S. Premature ejaculation: challenging new and the old concepts. F1000Res. 2017;6:2084. doi:10.12688/f1000research.12150.1
- Castiglione F, Albersen M, Hedlund P, Gratzke C, Salonia A, Giuliano F. Current pharmacological management of premature ejaculation: a systematic review and meta-analysis. Eur Urol. 2016;69(5):904-916. doi:10.1016/j.eururo.2015.12.028
- Waldinger MD, Zwinderman AH, Olivier B. On-demand treatment of premature ejaculation with clomipramine and paroxetine: a randomized, double-blind fixed-dose study with stopwatch assessment. Eur Urol. 2004;46(4):510-515; discussion 516. doi:10.1016/j.eururo.2004.05.005

