Clinical Overview: Rivaroxaban (Xarelto) for Multiple Cardiovascular Conditions
Rivaroxaban is the only FDA-approved anticoagulant used in patients with peripheral artery disease and coronary artery disease.
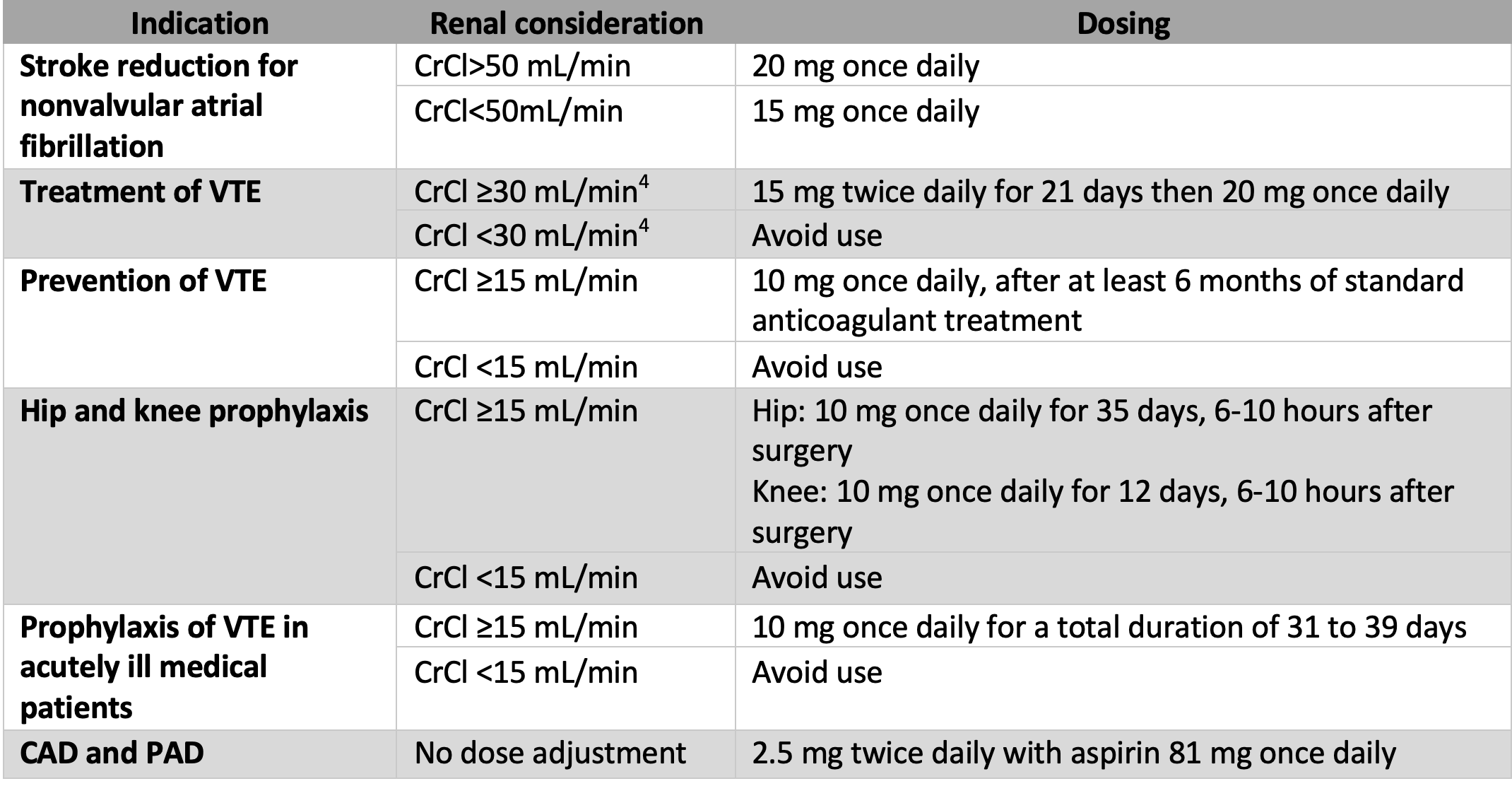
Rivaroxaban (Xarelto) is an anticoagulant approved for multiple conditions, such as nonvalvular atrial fibrillation, treatment and prevention of venous thromboembolism, and other indications categorized in Table 1.
Similar to other direct factor Xa inhibitors, rivaroxaban is an oral drug given at a fixed dose without routine monitoring.1 Although anticoagulants prevent venous thrombosis, rivaroxaban has a unique indication of use. It is the only FDA-approved anticoagulant used in patients with peripheral artery disease (PAD) and coronary artery disease (CAD).
In this population, rivaroxaban reduces the risk of heart attack, stroke, cardiovascular death, and major adverse limb events (MALE) when taken with 81 mg of aspirin.2
Table 1: Indication/Dosing for Rivaroxaban3
Venous thromboembolism (VTE) occurs when there is endothelial damage, hypercoagulability, or stasis of blood.5 These 3 conditions, known as Virchow’s triad, can result in deep vein thrombosis or pulmonary embolism.
Rivaroxaban works by directly inhibiting free and clot bound factor Xa in a dose-dependent manner. This inhibition prevents fibrin formation and reduces VTE recurrence.
In a meta-analysis evaluating the recurrence rate of DVT in patients receiving rivaroxaban, 258 of 19,144 (1.35%) patients had VTE within 6 months, displaying rivaroxaban’s efficacy in venous clot prevention.6 Treating PAD is complex because there are multiple factors that influence atherothrombosis.
Arterial clots happen when exposed plaque interacts with platelet receptors and coagulation factors, which leads to platelet aggregation.7 Decreasing thrombin generation and platelet aggregation with rivaroxaban and aspirin is one proposed mechanism to reduce arterial clots.
Prior to rivaroxaban, the recommended treatment for symptomatic patients with PAD was dual antiplatelet therapy (DAPT). In the PEGASUS trial, ticagrelor and aspirin demonstrated an absolute risk reduction of coronary events by 4.1% (NNT 25)9 and a reduction in all-cause mortality; however, it did not significantly reduce the risk of limb ischemia.9
This combination resulted in minimal bleeding episodes and is reasonably safe to use in this population.9 DAPT may not be appropriate for all patients. For example, patients with stable PAD, defined as those with PAD without myocardial infarction or stroke, benefit from single antiplatelet therapy with clopidegral or ticagrelor.
DAPT in chronic stable PAD may result in a higher risk of bleeds as evidenced by a study comparing clopidogrel and aspirin to aspirin alone, which resulted in significant bleeding [risk ratio (RR) 1.73, P= 0.004].10 The CAPRIE trial evaluated patients with stable PAD.
Clopidogrel was associated with a 22% reduction versus aspirin in the relative risk of major adverse coronary events,11 ticagrelor also proved non-superior to clopidegral for use in this population based off the EUCLID trial.12 DAPT can have a significant risk of bleeding and defining the proper patient population for appropriate utilization is difficult.
Rivaroxaban is an alternative to DAPT for patients with symptomatic PAD because it reduces the reoccurrence of cardiovascular events and MALE. Patients taking rivaroxaban 2.5 mg twice daily with aspirin 81 mg had a lower risk for composite death, stroke, and myocardial infarction compared to aspirin alone (p<0.001).12 Rivaroxaban treatment also reduced all-cause mortality by 18%.13
A subset study for symptomatic PAD noted an absolute risk reduction of MALE, including amputation at 30 months, by 4.2%.14 Although an increased risk of bleeding with this combination regimen was noted, organ and fatal bleedings were low, showing that the benefit of the medication outweighed the risk of bleeding.14
Compared to ticagrelor and aspirin, rivaroxaban and aspirin have the added benefit of reducing MALE. Both of these medications are beneficial for patients with comorbidities such as kidney disease, diabetes, increased age, and multi-vessel disease.9,12,14
In conclusion, there are many indications and uses for rivaroxaban. The 2.5 mg dose administered twice daily is beneficial for high-risk patients with PAD and CAD and is a reasonable alternative to DAPT.
References
- Antoniou S. Rivaroxaban for the treatment and prevention of thromboembolic disease. Journal of Pharmacy and Pharmacology. 2015;67(8):1119-1132. doi:10.1111/jphp.12387
- Kaplovitch E, Eikelboom JW, Dyal L, et al. Rivaroxaban and aspirin in patients with symptomatic lower extremity peripheral artery disease. JAMA Cardiology. 2020. doi:10.1001/jamacardio.2020.4390
- Xarelto (rivaroxaban) tablets label - food and drug .https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202439s001lbl.pdf.
- Aursulesei V, Costache II. Anticoagulation in chronic kidney disease: From guidelines to clinical practice. Clinical Cardiology. 2019;42(8):774-782. doi:10.1002/clc.23196
- Aleman MM, Walton BL, Byrnes JR, Wolberg AS. Fibrinogen and red blood cells in venous thrombosis. Thromb Res. 2014;133 Suppl 1(0 1):S38-S40. doi:10.1016/j.thromres.2014.03.017
- Aryal MR, Gosain R, Donato A, et al. Systematic review and meta-analysis of the efficacy and safety of apixaban compared to rivaroxaban in acute VTE in the real world. Blood Adv. 2019;3(15):2381-2387. doi:10.1182/bloodadvances.2019000572
- Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med. 2014;276(6):618-632. doi:10.1111/joim.12296
- Hiatt WR, Fowkes FG, Heizer G, et al. Ticagrelor versus Clopidogrel in Symptomatic Peripheral Artery Disease. N Engl J Med. 2017;376(1):32-40. doi:10.1056/NEJMoa1611688
- Bonaca MP, Bhatt DL, Storey RF, et al. Ticagrelor for prevention of ischemic events after myocardial infarction in patients with peripheral artery disease. Journal of the American College of Cardiology. 2016;67(23):2719-2728. doi:10.1016/j.jacc.2016.03.524
- Gajulapalli RD, Dias S, Pattanshetty DJ, Athappan G. Optimal duration of dual antiplatelet therapy after drug eluting stent implantation: a network meta-analysis. Anatol J Cardiol. 2017;18(4):251-260. doi:10.14744/AnatolJCardiol.2017.7672
- Creager MA. Results of the CAPRIE trial: efficacy and safety of clopidogrel. Clopidogrel versus aspirin in patients at risk of ischaemic events. Vasc Med. 1998;3(3):257-260
- Hiatt WR, Fowkes FG, Heizer G, et al. Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. New England Journal of Medicine. 2017;376(1):32-40. doi:10.1056/nejmoa1611688
- Steffel J, Eikelboom JW, Anand SS, Shestakovska O, Yusuf S, Fox KAA. The compass trial. Circulation. 2020;142(1):40-48. doi:10.1161/circulationaha.120.046048
- Kaplovitch, E., Eikelboom, J. W.,et al. Rivaroxaban and Aspirin in Patients With Symptomatic Lower Extremity Peripheral Artery Disease: A Subanalysis of the COMPASS Randomized Clinical Trial. JAMA cardiology, (2021). 6(1), 21–29. https://doi.org/10.1001/jamacardio.2020.4390
