Top biopharmaceutical Covid-19 vaccine companies boosted with over 80% revenue growth
Pharmaceutical Technology
SEPTEMBER 15, 2022
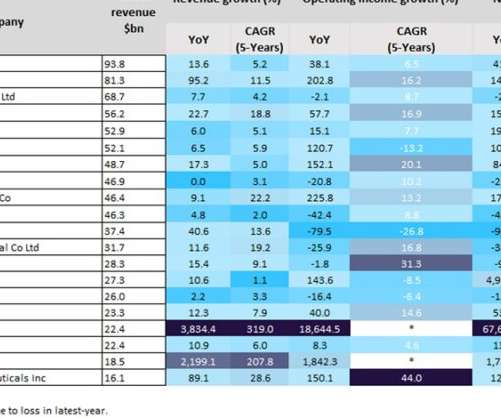
Last year was a positive year for biopharmaceutical companies, particularly those with Covid-19 vaccines. As a result of huge global sales of mRNA Covid-19 vaccines, the split in profits between Pfizer and BioNTech’s Comirnaty contributed towards revenues of $81.3bn and $22.4bn last year, respectively. YoY revenue growth.
































Let's personalize your content