Preventing fungal contamination in pharmaceuticals
European Pharmaceutical Review
MARCH 11, 2024
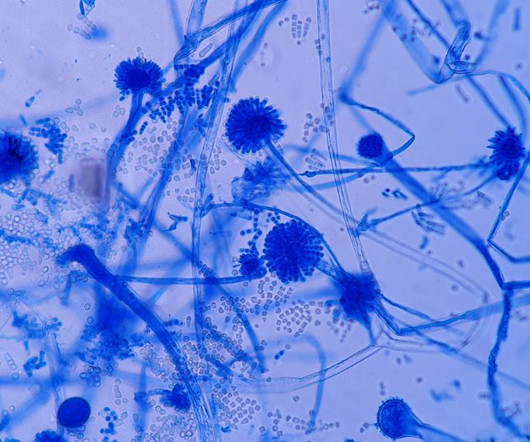
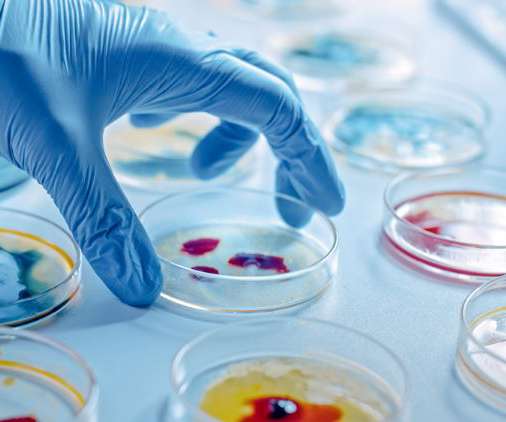
In a review on fungal-contaminated compounded pharmaceuticals and medical devices, researchers have described how the contamination of these products can be due to breaches in sterile compounding procedures. Ahmed et al. Ahmed et al. One case of drug contamination from 2021 was highlighted in the paper.













Let's personalize your content