Smart pharmaceutical and healthcare labels: Lets trace medicines from its origin
Roots Analysis
MARCH 8, 2023
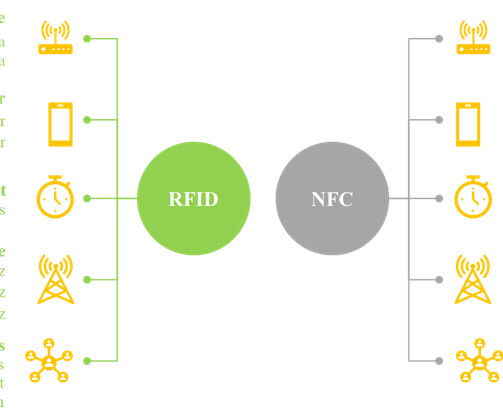
In recent years, the use of smart labels allows the developer to convey a greater amount of information about the product to the consumers, without the need for additional packaging space. Further, smart labels play a critical role in the manufacturing of pharmaceuticals.


























Let's personalize your content