FDA cracks down on off-label drug use messaging
Pharmaceutical Technology
OCTOBER 24, 2023
The FDA released a draft guidance giving firms recommendations on provider-directed communication for off-label drug use.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

Pharmaceutical Technology
OCTOBER 24, 2023
The FDA released a draft guidance giving firms recommendations on provider-directed communication for off-label drug use.
Roots Analysis
MARCH 8, 2023
In recent years, the use of smart labels allows the developer to convey a greater amount of information about the product to the consumers, without the need for additional packaging space. Further, smart labels play a critical role in the manufacturing of pharmaceuticals.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

The FDA Law Blog
MAY 11, 2023
GSK skinny label case , the U.S. Specifically, the Government explained, “[t]he section viii pathway cannot function properly if FDA and generic manufacturers cannot rely on an NDA holder’s representations to the agency regarding which portions of the brand-name drug’s labeling teach patented methods of use.”

The FDA Law Blog
APRIL 25, 2024
Promotional labeling is generally any labeling other than FDA-required labeling that is devised for the promotion of a product, as well as other functions, and can include printed, audio, or visual matter that describes the product. l)(1) (e.g.,

BuzzRx
JANUARY 27, 2023
The term “on-label use” of a drug may seem unfamiliar to most people. In short, this practice is referred to as “off-label” drug use. Surveys have shown that approximately 1 in 5 prescriptions in the US are for off-label use. In certain populations of patients, off-label drug use is even higher.
The Checkup by Singlecare
JANUARY 26, 2023
If you’ve worked in a community pharmacy, you’ve likely had the same frustrating conversations about prescription labels over and over. Due to variability in health literacy, studies show that Americans’ level of understanding of prescription label instructions ranges from 53% to 89%.

The FDA Law Blog
AUGUST 21, 2023
Livornese — I saw the sign…and the answer is no—FDA-approved labeling apparently is not enough under state failure-to-warn laws, according to certain courts.

The FDA Law Blog
OCTOBER 24, 2023
By Dara Katcher Levy — Yesterday, FDA published a new Draft Guidance, “ Communications from Firms to Health Care Providers Regarding Scientific Information on Unapproved Uses of Approved/Cleared Medical Products Questions and Answers ” (SIUU Guidance or Draft Guidance).

PharmExec
JULY 7, 2023
Newly-issued final guidance focuses on language used when communicating quantitative efficacy or risk information.
STAT
AUGUST 25, 2023
Many see these models as assistants or even potential replacements for time-intensive tasks, like patient-physician communication through the electronic health record. As a result, they have been labeled “foundation models.

The FDA Law Blog
AUGUST 23, 2022
Ironically, a recent FDA safety communication points to a potential way out of this dilemma. In this safety communication, FDA advises the public as follows: If you test negative and have COVID-19 symptoms , you test again 48 hours later for a total of two tests.

Pharma Marketing Network
AUGUST 9, 2023
Navigating these regulatory challenges is essential to ensure compliance, maintain trust, and effectively communicate the benefits and risks of pharmaceutical products. Develop Clear and Balanced Messaging Effective communication is at the heart of pharmaceutical marketing. Keep thorough records of approvals for future reference.

Pharma Marketing Network
AUGUST 9, 2023
Navigating these regulatory challenges is essential to ensure compliance, maintain trust, and effectively communicate the benefits and risks of pharmaceutical products. Develop Clear and Balanced Messaging Effective communication is at the heart of pharmaceutical marketing. Keep thorough records of approvals for future reference.

Pharmaceutical Technology
APRIL 17, 2023
However, the treatment’s label features a black box warning that includes increased mortality in elderly patients with dementia-related psychosis treated with antipsychotic drugs. He added that there is also a need for FDA-approved products that communicate efficacy and safety on their labels.

Pharmafile
FEBRUARY 28, 2023
Johnson & Johnson (J&J)’s Janssen-Cilag unit has announced that its fixed-dose combination drug Akeega has been recommended for approval by the EMA’s Committee for Medicinal Products for Human Use (CHMP) for patients with metastatic castration-resistant prostate cancer (mCRPC), but that it should be limited to patients with BRCA1/2 mutations. (..)

The Checkup by Singlecare
MARCH 31, 2024
Patients should carefully review medication labels and consult healthcare professionals to understand potential side effects before driving. In addition, you should read labels carefully, understand medication interactions, and plan for safe transportation. Openly share your concerns and experiences to receive personalized guidance.

pharmaphorum
DECEMBER 7, 2020
Wokingham, United Kingdom — 7 December 2020 — PRISYM ID, a leading provider of regulated content and label management solutions for the life sciences sector, announced today that PRISYM 360 version 1.10 PRISYM 360 and SAP technologies communicate through a web service using standard SAP components with no intermediate stages.

pharmaphorum
OCTOBER 27, 2022
The overarching principle set out in Codes of Practice, and in particular the Principles for the use of digital channels in the EFPIA Code , is that the legislation and Codes of Practice apply equally to communications by companies on social media and digital channels.
pharmaphorum
JULY 20, 2021
The US regulator had extended its review of the drug by three months – setting back its action date from April to 29 July – but now says that deficiencies in the marketing application “preclude discussion” of labelling and post-marketing requirements.

Viseven
NOVEMBER 17, 2022
Medical affairs in Pharma are often seen as a central agency that works within a healthcare company and prioritize communication among life science organizations, medical professionals, healthcare providers, and patients. Medical affairs definition uses clinical and scientific information to communicate the efficiency of a drug.

pharmaphorum
NOVEMBER 29, 2022
Both patients were in an open-label extension of the trial, so it is known that they were on the drug at the time of death, though they may have been in the placebo group previously. The second death, reported by Science earlier this week, concerned a 65-year-old woman who also died of a brain haemorrhage.
pharmaphorum
SEPTEMBER 29, 2022
Effective communication is vital for addressing […] barriers, and language is one of the key tools we use to communicate. Despite this, non-inclusive words, phrases, and labels are often used in scientific publications and clinical trial protocols. Barriers to health. Equitable health.

Pharmaceutical Technology
JULY 20, 2022
Ganio says that, ideally, once an individual receives a positive Covid-19 result, they should call their pharmacy or communicate through a drive-through facility to minimize the risk of exposure. The US FDA has not announced any updates to Lagevrio’s label. How to get Paxlovid.

Pharmaceutical Technology
JULY 29, 2022
Dr Jeremy Veenstra-VanderWeele, professor of developmental neuropsychiatry at the Columbia University Irving Medical Center, explains that agitation is seen in the minority of autistic teens, who struggle with communication. An inability to clearly communicate with others and express their wants and needs results in frustration, he adds.

The Checkup by Singlecare
MAY 23, 2023
Though most commonly prescribed to treat depression, it is sometimes prescribed off-label for attention-deficit/hyperactivity disorder (ADHD) or (the 12-hour extended-release form) smoking cessation. It is also sometimes prescribed off-label for ADHD or smoking cessation. Not all antidepressants work the same way.
Pharmaceutical Technology
MARCH 22, 2023
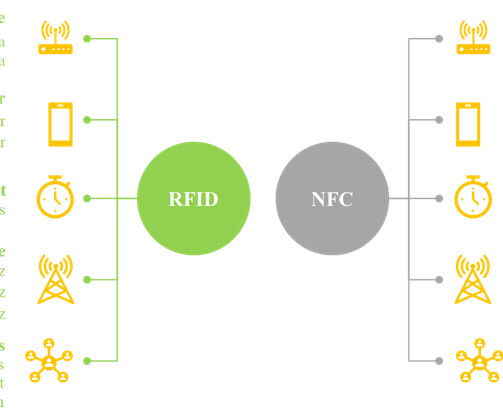
What lies ahead for RFID and smart labelling Volpe says “the future is bright” for smart technologies that identify, monitor, and track medications through the supply chain. RFID is an important facet of smart labelling and its evolution, but not the only one.

pharmaphorum
MARCH 30, 2021
Such interventions take the need for robust value claims to a completely different level, with important implications for the evidence companies need to collect, and how they collect and communicate it. There are a number of drivers behind the need to rethink value evidence and value communication. Changes are afoot – what to look for.
pharmaphorum
AUGUST 24, 2020
In the United States, the 21st Century Cures Act encouraged the Food and Drug Administration (FDA) to review and communicate patient experience data from trials – but the lack of a common framework for submissions and space on product labels has, until now, been something of a stumbling block. .

pharmaphorum
OCTOBER 7, 2021
Rewind to March of 2020, and pharma companies were facing a communication crisis. Other uses include providing a platform for key opinion leaders (KOLs), as well as internal communications to make sure that messages are unified across an organisation. The crisis that changed everything.
pharmaphorum
FEBRUARY 5, 2021
After the ORAL Surveillance data emerged, analysts debated whether the FDA might take action either to remove Xeljanz from the market or – perhaps more likely – update its labelling to reflect the new data and leave prescribing decisions to doctor discretion.

pharmaphorum
MAY 25, 2022
Where it is impossible to restrict access to HCPs, due to the congress platform or equivalent, there must be a clear statement to the attendee that the materials/communication are designed and intended for HCPs only.”. Identifying the appropriate code and label. Here, we take a look at the top five takeaways from the document: 1.

The FDA Law Blog
NOVEMBER 29, 2023
Considerations include transparency regarding the data used to develop the change, comprehensive testing of the change, characterizing the performance of the device before and after the change, and plans in place for ongoing monitoring of device performance and communication of any unexpected changes in performance.

The Checkup by Singlecare
NOVEMBER 30, 2023
Available under the brand names Neurontin , Gralise , and Horizant , gabapentin is also commonly prescribed off-label for general nerve pain, migraines , and bipolar disorder. Off-label use is when a drug is used for a purpose other than what it is approved for. In some cases, these may be related to drug interaction.

pharmaphorum
AUGUST 27, 2020
Bioindustry association MichBio says the FDA’s labelling requirements for COVID-19 convalescent plasma (CCP) – which were published alongside the emergency use authorisation – could lead to “hundreds, if not thousands, of in-date, ready to transfuse CCP units across the country being rendered unusable.”.

BuzzRx
DECEMBER 8, 2022
Read the product label and follow the prescription’s instructions carefully. What is the FDA drug safety communication regarding codeine cough medicine? In the same FDA drug safety communication, the agency also recommends against the use of these prescription medicines in breastfeeding mothers due to possible harm to their infants.

pharmaphorum
OCTOBER 12, 2018
The development of software applications that are available with a prescription took a major step forward last year with the first FDA approval for a mobile medical application with both a safety and efficacy label.

Digital Pharmacist
MAY 25, 2023
Properly organizing medication bottles in a systematic manner where the label is visible may be a good idea. Consider Allergies Communicate with patients to record any known drug allergies in the system. It’s important to ensure that they are either stored away from each other or that there is a distinct way to differentiate them.

The Checkup by Singlecare
OCTOBER 16, 2023
What Are the Off-Label Uses for Cymbalta? Cymbalta may have several off-label uses in addition to its FDA-approved uses. Migraine Headaches Another off-label use of Cymbalta is for the management of migraine headaches. The medication may help strengthen the muscles that control urine flow and improve overall bladder function.
The Checkup by Singlecare
MARCH 8, 2024
It is also used off-label for other forms of anxiety, like agoraphobia, panic disorders, and acute vertigo episodes. An FDA drug safety communication described that combining opioids with benzodiazepines like alprazolam has resulted in serious adverse events related to the additive CNS depression contributed by both types of medication.
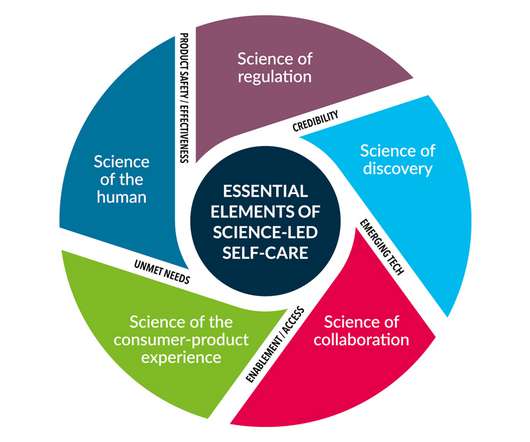
pharmaphorum
SEPTEMBER 6, 2022
The first principle, labelled the “Science of the Human”, asserts that self-care products should be rooted in a thorough understanding of human biology and medical insights, while the second, the “Science of Regulation”, calls for independent regulation.

The Checkup by Singlecare
MARCH 16, 2023
This includes counting, measuring, and packaging medications, as well as labeling and organizing them for distribution. “They work closely with pharmacists to ensure that medications are prepared and dispensed accurately and safely,” he says. RELATED: Ways to prevent common pharmacy errors 7.

Digital Pharmacist
DECEMBER 6, 2022
Properly organizing medication bottles in a systematic manner where the label is visible may be a good idea. Communicate with patients to record any known drug allergies in the system. It’s important to ensure that they are either stored away from each other or that there is a distinct way to differentiate them. Verify Orders.

IDStewardship
JUNE 6, 2023
Streamlined Prescription Filling : AI systems can automate prescription filling processes by extracting relevant information from electronic prescriptions, verifying insurance coverage, and generating labels. AI can assist in language translation, improving communication with non-native speakers.

The Checkup by Singlecare
NOVEMBER 11, 2023
Communication is the second necessary precaution. When to talk to a healthcare provider about Imodium interactions Part of the key to good communication concerning safety in healthcare is being willing to speak up. Keep a list of prescription drugs , over-the-counter drugs, and supplements.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content