RFID: The future of smart labelling?
Pharmaceutical Technology
MARCH 22, 2023
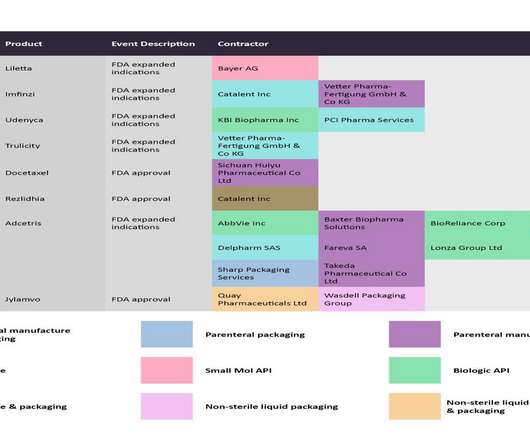
In the US Drug Quality and Security Act (DSCSA), the FDA mandates that manufacturers and trading partners should have full interoperable electronic track and trace systems in place by November 2023. RFID is an important facet of smart labelling and its evolution, but not the only one.







































Let's personalize your content