5 Things To Know About Antimicrobial Stewardship Regulatory Standards
IDStewardship
APRIL 13, 2023
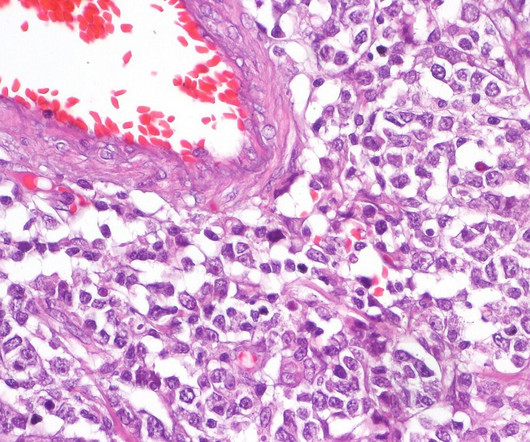
PACCARB provides advice, information, and recommendations to the Health and Human Services Secretary (HHS) regarding programs and policies intended to support and evaluate the implementation of U.S. CMS regulations on antimicrobial stewardship for acute care ( QSO-22-20-Hospitals ) went into effect mid-2022.












































Let's personalize your content