Analytica 2024 in overview
European Pharmaceutical Review
MARCH 5, 2024
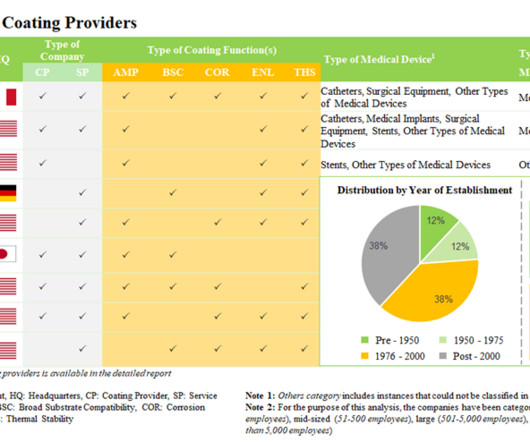
International participation and trending topics With over 900 exhibitors, analytica 2024 promises an impressive lineup. Artificial intelligence (AI) plays an increasingly important role in the laboratory world, especially in medical diagnostics. Top topics at the trade fair include digitalisation, Laboratory 4.0,















































Let's personalize your content