PharmaShots Weekly Snapshots (May 08 - 12, 2023)
PharmaShots
MAY 12, 2023
Sanofi Reports P-IIIb Trial (HARMONIE) Results of Nirsevimab for the Prevention of Hospitalizations due to RSV-Related LRTD Date: May 12, 2023 | Tags: Sanofi, Nirsevimab, RSV-Related LRTD, Clinical Trial, P-IIIb, HARMONIE Trial G1 Therapeutics Presents Preliminary Results from P-II Trial of Trilaciclib for Triple-Negative Breast Cancer at ESMO 2023 (..)






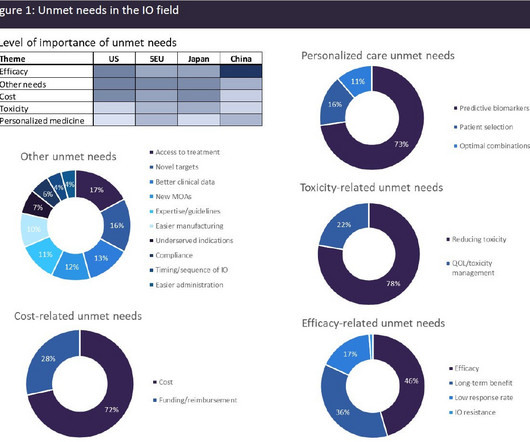







Let's personalize your content