STAT+: The FDA has approved a new Alzheimer’s drug, but wide access may depend on CMS easing restrictions
STAT
JANUARY 6, 2023
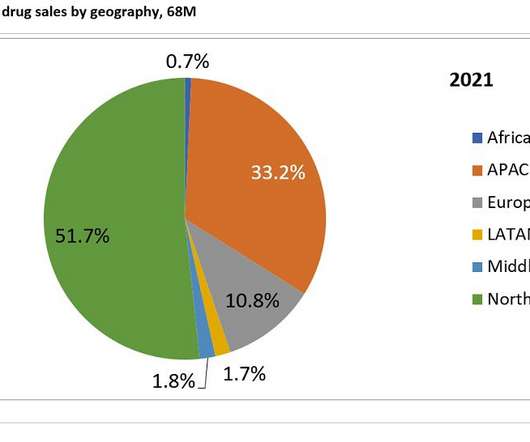
Such decisions are based on myriad factors, starting with the average $26,500 price tag. But there are other considerations, including the quality of the clinical trial data, side effect concerns, the patient population for which the medicine is approved, and budgetary constraints.



































Let's personalize your content