Nine for 2021: Addressing the pandemic legacy
pharmaphorum
JANUARY 14, 2021
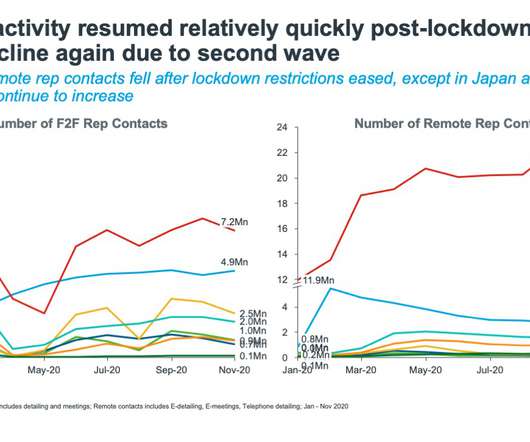
Not so China, and especially not so the lead five European countries – on average European pharmaceutical companies saw a loss of 30% of the interactive time they previously had with healthcare professionals in 2020. Whatever the challenges, the pharmaceutical industry enters 2021 with a new sense of purpose. Pharma pivots East.









Let's personalize your content