Lannett sells discontinued generics, inks private label deal for others
Drug Store News
OCTOBER 19, 2022
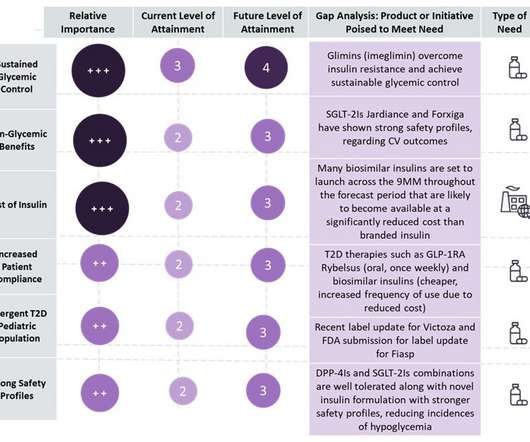
Lannett's deal call for another pharmaceutical company to purchase and distribute under a private label a limited number of certain other Lannett owned generic drug products.









































Let's personalize your content