Merck’s Keytruda wins coveted FDA nod around surgery for early lung cancer—with a surprise
Fierce Pharma
OCTOBER 16, 2023
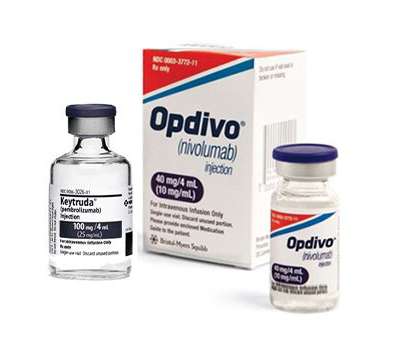
Up until this point, immune checkpoint inhibitors have been allowed to treat early-stage non-small cell lung cancer (NSCLC) either before or after surgery. Thanks to a new FDA approval for Merck's Keytruda, a continuous immunotherapy regimen around surgery is now available for patients with early-stage non-small cell lung cancer.





































Let's personalize your content