FDA Approval of Whooping Cough Vaccine for Infants Less Than 2 Months of Age
Digital Pharmacist
OCTOBER 26, 2022
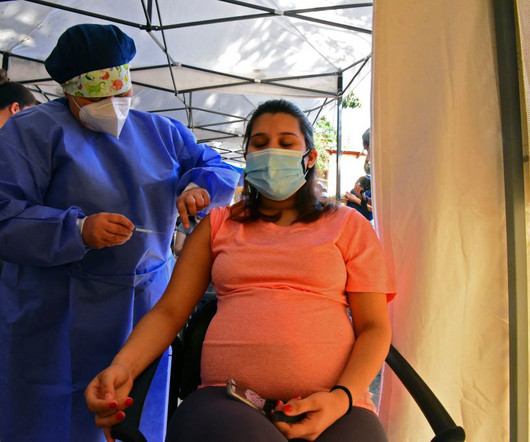
Food and Drug Administration (FDA) announced its approval of the Boostrix vaccine, commonly known as Tdap (combination of Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis) for immunization administration during the third trimester of pregnancy to prevent pertussis in infants younger than two months of age.































Let's personalize your content