Digital health tech: a solution to substance use disorders?
Pharmaceutical Technology
JULY 4, 2023
Substance use disorders leave people with long-term negative mental and physical health implications and can lead to death.

Pharmaceutical Technology
JULY 4, 2023
Substance use disorders leave people with long-term negative mental and physical health implications and can lead to death.
European Pharmaceutical Review
JULY 4, 2023
The International Organization for Standardization (ISO) has published its new standard Sterilization of health care products — Microbiological methods — Part 3 Bacterial endotoxin testing ( ISO 11737-3:2023). The document contains requirements and guidance for testing for bacterial endotoxins. This includes products that must be non-pyrogenic based on either intended use or non-pyrogenic label claim, or both.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Outsourcing Pharma
JULY 4, 2023
Drugs like Annovis Bioâs buntanetap and prasinezumab have the potential to ârevolutionize the treatment of Parkinsonâs Diseaseâ says analytics company, GlobalData, an analytics company, but it is a complex road ahead.

The Honest Apothecary
JULY 4, 2023
Jeremy, an HR business partner to the nursing staff of a medium sized community hospital, arrived at his desk at 8:30am on Monday to a familiar sight. There, sitting on his desk, was yet another resignation letter from a nurse on the 7th floor. Tina’s floor. Over the past 6 months Jeremy had 5 such letters reach his desk. Each one a subtle reminder to him that his organization is dealing face-to-face with the problem with toxic leadership in healthcare. “I regret to inform you that m

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.
Pharmaceutical Technology
JULY 4, 2023
While drug shortages have been a major cause for concern over the past few years, medications to treat cancer are in particularly short supply.
Pharma Tutor
JULY 4, 2023
Reality Test of Medicine Shop without Registered Pharmacist admin Tue, 07/04/2023 - 15:39 ABOUT AUTHOR Dr. R. S. Thakur Renowned Professor of Pharmaceutical Fraternity & Former Member of Pharmacy Council of India. Email : drramsthakur@gmail.
Pharmacy Technician Pulse brings together the best content for pharmacy technicians from the widest variety of industry thought leaders.
Pharmaceutical Technology
JULY 4, 2023
Abeona Therapeutics has raised $25m from its current select investors to launch and commercialise its cell therapy, EB-101.

European Pharmaceutical Review
JULY 4, 2023
As drug development professionals know, global health crises like the COVID-19 pandemic provide the public with valuable insights into how clinical research and regulatory processes work. However, critical disciplines within the field responsible for ensuring safe and effective drug products, including quality assurance and compliance, remain mysterious or invisible to many.

Outsourcing Pharma
JULY 4, 2023
Clerkenwell Health, a specialist clinical research organisation (CRO) focused on supporting clients with the design and delivery of psychedelic-assisted therapy trials, is sponsoring the upcoming Psych Symposium 2023 on Thursday 6 July in London.

European Pharmaceutical Review
JULY 4, 2023
Trends in capsule formulation In this Q&A, Recipharm’s Torkel Gren discusses developments in capsule formulation, including the shift away from gelatine and the potential for growth in the inhalation capsule market. Titanium dioxide: are there alternatives? Mike Tobyn from Bristol Myers Squibb, Jonathan Kaye from GSK, David Harris from MSD and Eli Lilly’s Jason Melnick discuss the role of E171 (titanium dioxide) in the identification of solid oral dosage forms.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.
pharmaphorum
JULY 4, 2023
Providing access to quality vaccines in low- and middle-income countries Mike.
European Pharmaceutical Review
JULY 4, 2023
The rise in antimicrobial resistance , 1 lack of significant antimicrobial discovery in recent years, and increasing instances of multidrug-resistant (MDR) microorganisms 2 have propelled the interest in bacteriophage (Phage) therapy as a potential new course of treatment. Patients with implantable devices are more prone to biofilm-mediated infections, 3 while other infections such as skin structure infections, chronic lung diseases resulting from respiratory infections, and urinary tract infect

pharmaphorum
JULY 4, 2023
Psychedelics and mental health: The time is now? Nicole.
European Pharmaceutical Review
JULY 4, 2023
JNJ-2113, the first and only oral interleukin-23 receptor (IL-23R) antagonist peptide in development for moderate-to-severe plaque psoriasis (PsO) has demonstrated positive results in a Phase II trial. Janssen’s Phase IIb FRONTIER 1 clinical trial for adult participants achieved all primary and secondary efficacy endpoints, according to topline results showcased by Bissonnette R, Pinter A, Ferris L, et al. at the World Congress of Dermatology 2023.

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.
pharmaphorum
JULY 4, 2023
Janssen celebrates positive results for JNJ-2113A in psoriasis trial Eloise.

NICE
JULY 4, 2023
The NHS touches all our lives. It treats over a million people a day in England, and was the first universal health system, available to all, free at the point of delivery. As we mark its 75th anniversary, I’m sure many of us will be taking some time to celebrate this remarkable institution. Over the years, one of the NHS’ key strengths has been its ability to adapt, and evolve, to meet the needs of successive generations.

pharmaphorum
JULY 4, 2023
Retaining the human quality of healthcare in DTx Mike.

Pharmaceutical Technology
JULY 4, 2023
Amneal follows a successful year of approvals with six more approvals for generics across multiple indications.

pharmaphorum
JULY 4, 2023
Worrying signs for trial diversity require broad industry action Mike.

Pharmaceutical Technology
JULY 4, 2023
The US FDA has issued a complete response letter to Amneal Pharmaceuticals, declining to approve its IPX203 to treat Parkinson’s disease.

pharmaphorum
JULY 4, 2023
So it begins: next Humira biosimilars launch in US Nicole.
Pharmaceutical Technology
JULY 4, 2023
Moderna has filed a regulatory application seeking EMA approval for its modified Covid-19 vaccine targeting the XBB.1.5 sub-variant.

Pharma Times
JULY 4, 2023
Therapy has been developed to reduce the frequency, duration and severity of migraine attacks - News - PharmaTimes

Pharmaceutical Technology
JULY 4, 2023
How can you reduce drop-out, save time and resources, and maintain participant engagement throughout the entirety of your trial?

Pharma Times
JULY 4, 2023
Collaboration aims to revitalise two empty sites on the South Bank of London by providing several laboratories - News - PharmaTimes
Pharmaceutical Technology
JULY 4, 2023
NorthX Biologics has acquired Valneva’s clinical trial manufacturing unit in Stockholm, Sweden to bolster its expertise.

The FDA Law Blog
JULY 4, 2023
By Deborah L. Livornese — On June 23, 2023, FDA issued a draft guidance for industry – Formal Dispute Resolution and Administrative Hearings of Final Administrative Orders Under Section 505G of the Food, Drug, and Cosmetic Act (the Monograph FDR Guidance) – to fulfill another commitment agreed to in support of the 2020 Coronavirus Aid, Relief, and Economic Security Act (CARES Act)(see our blog posts here , here and here about the CARES Act and OTC monograph reform).
Pharmaceutical Technology
JULY 4, 2023
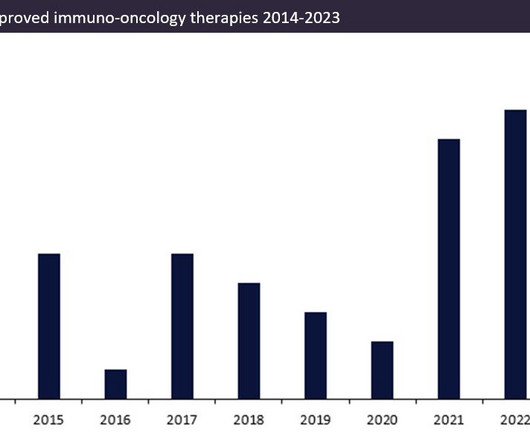
Over the last ten years, the number of approved innovator immuno-oncology therapies (immunotherapies) has increased.

Fierce Healthcare
JULY 4, 2023
The Biden administration issued a proposal Friday to cut reimbursements to home health providers by 2.2% next year, or an estimated $375 million less than 2023 payment levels. | The Biden administration issued a proposal Friday to cut reimbursements to home health providers by 2.2% next year, or an estimated $375 million less than 2023 payment levels.
Pharmaceutical Technology
JULY 4, 2023
The venous thromboembolism (VTE) market is expected to grow from $3.6bn to $4.6bn globally, according to GlobalData.

Pharmaceutical Technology
JULY 4, 2023
Tonix Pharmaceuticals and Tonix Medicines have acquired two migraine products from Upsher-Smith Laboratories.

Pharmaceutical Technology
JULY 4, 2023
The spin-off will create a new global Phase I-IV CRO, patient access and technology solutions.

Pharmaceutical Technology
JULY 4, 2023
The gene therapy prevents neovascularisation from a single, intravitreally delivered injection.
Let's personalize your content