FDA Accepts Biologics License Application for Proposed Denosumab Biosimilar
Drug Topics
MARCH 8, 2023
The drug, manufactured by Sandoz, will treat conditions including osteoporosis in postmenopausal women
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

 condition osteoporosis
condition osteoporosis 
Drug Topics
MARCH 8, 2023
The drug, manufactured by Sandoz, will treat conditions including osteoporosis in postmenopausal women

The Checkup by Singlecare
MARCH 13, 2024
Osteoporosis and bone density conditions are common in the U.S.—the According to a 10-year review of clinical studies , it is a highly effective anti-osteoporosis therapy for patients at high risk of fracture. Like Prolia, Jubbonti must be given as an injection by healthcare providers every six months.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Drug Store News
FEBRUARY 14, 2023
Denosumab is indicated for treating variety of conditions, such as osteoporosis in postmenopausal women.
pharmaphorum
OCTOBER 13, 2022
It is World Osteoporosis Day this month, and Ben Hargreaves takes a look at what treatment options look like for patients living with the disease. Osteoporosis is a condition that causes bones to become weak and fragile, making them more likely to break due to a fall or even something as minor as a sudden movement.
Pharmaceutical Technology
FEBRUARY 7, 2023
Denosumab will be used to treat several conditions, including osteoporosis in postmenopausal women and in men with high fractures risk, treatment-induced bone loss. This results in bone loss reduction and the likelihood of fractures and other serious conditions related to bones.
pharmaphorum
APRIL 1, 2022
NICE has reversed its stance on UCB and Amgen’s severe osteoporosis therapy Evenity, saying out can be prescribed on the NHS for women with the disease in England and Wales who are at high risk of fractures. ” “Osteoporosis is one of the most urgent challenges to people living well in later life,” he added.

Pharmaceutical Technology
MAY 26, 2023
The MAAs include all indications covered by the Xgeva (denosumab) and Prolia (denosumab) reference medicines to treat conditions such as osteoporosis in postmenopausal women. Other conditions include the prevention of skeletal-related complications in cancer that has spread to the bones, as well as treatment-induced bone loss.
European Pharmaceutical Review
JUNE 19, 2023
We believe this study provides the first clinical evidence of safe and successful delivery of the osteoporosis drug teriparatide through an oral robotic pill,” stated Arvinder Dhalla, Vice President of Clinical Development at Rani Therapeutics, which was the company that developed the technology.

Pharmacy Is Right For Me
JANUARY 3, 2024
In her role at the Providence Family Medicine Center, she dedicates her time to assisting clinic residents with medication treatment plans and conducts individual visits with patients, prescribing medications for chronic conditions such as diabetes, hypertension, osteoporosis, asthma, and COPD. Beyond medical treatment, Dr.
pharmaphorum
OCTOBER 20, 2021
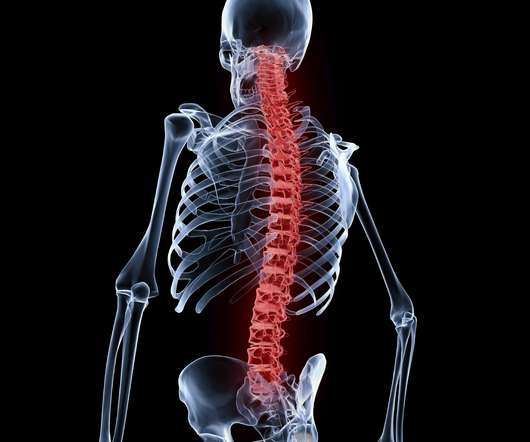
The digital health tool, known as BoneBot, applies machine learning to X-rays to detect ‘silent’ or asymptomatic fractures in the vertebrae of the spine, a feature of the bone-wasting disease osteoporosis that can remain undiagnosed in more than two-thirds of cases.

European Pharmaceutical Review
NOVEMBER 14, 2022
Four medicines recommended for approval in the European Medicines Agency (EMA)’s Committee for Medicinal Products for Human Use (CHMP)’s latest meeting included a biosimilar for osteoporosis and a novel COVID-19 vaccine. CHMP’s safety update.

Med Ed 101
JULY 19, 2020
Osteoporosis is the condition in which bone density is reduced and the patient has an increased risk of fractures. According to the International Osteoporosis Foundation, an estimated 44 million people in the US population aged 50 and older suffer from osteoporosis and low bone mass.
The Checkup by Singlecare
DECEMBER 20, 2023
Millions of people are prescribed prednisone each year for conditions that range from psoriasis and eczema to multiple sclerosis and rheumatoid arthritis. For individuals living with an inflammatory illness or condition, corticosteroids such as prednisone can feel like a godsend. The benefits of prednisone also come with risks.

The Checkup by Singlecare
APRIL 14, 2024
Prednisone is a steroid medication that is used to treat various conditions that involve inflammation, such as severe allergies , severe skin conditions , ulcerative colitis , multiple sclerosis , rheumatoid arthritis , and many others. The dosage of prednisone varies depending on individual factors and medical conditions.

The People's Pharmacy
APRIL 27, 2023
There are a number of drugs that are used to combat osteoporosis, which is certainly a benefit. As a result, people who smoke and have osteoporosis may need to consult their doctors about other treatment options. My doctor is recommending Reclast to treat osteoporosis. Could Reclast Side Effects Cause Dental Problems?

Med Ed 101
DECEMBER 12, 2021
Bisphosphonates are frequently used in the management of osteoporosis. They are often the go-to agent for this condition. Below we provide a bisphosphonate comparison table and I also wanted to highlight some of the most important clinical pearls that we should teach our patients and colleagues.

The Checkup by Singlecare
OCTOBER 16, 2023
If you or a loved one have recently been injured or diagnosed with an illness that impedes your daily life, you might wonder: What medical conditions automatically qualify you for disability? This means you must develop a severe medical ailment on the list of disabling conditions and then meet other criteria for each type of benefit.
The Checkup by Singlecare
MARCH 1, 2024
Food and Drug Administration (FDA) to treat various inflammatory conditions , such as asthma , severe allergies , rheumatoid arthritis , ulcerative colitis , and blood disorders. Dexamethasone and certain medical conditions There are some contraindications (situations where it is not safe to take a medication) associated with dexamethasone.

The Checkup by Singlecare
DECEMBER 6, 2023
It is one of the most commonly used medications that can treat gastroesophageal reflux disease (GERD) and other stomach acid-related conditions. Communicating with the healthcare team about all medical conditions and medications is key to avoiding this drug interaction. An important interaction to be aware of is with the supplement St.

Pharmaceutical Technology
MARCH 10, 2023
Denosumab is indicated for treating various conditions, including osteoporosis in postmenopausal women and men with high fracture risk and treatment-induced bone loss. Last month, Sandoz announced that the US Food and Drug Administration (FDA) accepted its biologics license application (BLA) for its proposed biosimilar denosumab.

pharmaphorum
JANUARY 11, 2023
For the Spring 2023 cohort, PharmStars will select startups with digital innovations that promote more equitable and inclusive healthcare for underserved population or focus on women’s health conditions (e.g.,

The Checkup by Singlecare
SEPTEMBER 7, 2023
Step therapy focuses on using cost-effective treatments for various conditions, which may help save money in the long run for insurance companies and the people they cover. In step therapy, the first-line alternatives are commonly generic drugs proven effective and safe for treating specific medical conditions.

Pharmaceutical Technology
MAY 30, 2023
The Italian pharmaceutical company Abiogen Pharma completed an acquisition of a 97.09% stake in the Swiss orphan conditions-focused EffRx Pharmaceuticals on May 29. The company developed and launched Binosto (alendronate sodium) as the first and only buffered solution for osteoporosis. Alkindi is one of EffRx’s three marketed drugs.

The Checkup by Singlecare
JULY 27, 2023
Health conditions like acid reflux, ADHD, and allergies have many medication options available. Use our drug comparison guides to compare Rx and OTC uses, doses, side effects, interactions, and prices.

The Checkup by Singlecare
OCTOBER 20, 2023
However, chronic inflammation can contribute to many medical conditions, including arthritis , diabetes , atherosclerosis, and neurological diseases. Since shilajit has anti-inflammatory properties, it may help reduce the risk of these and other inflammatory conditions.
The Checkup by Singlecare
AUGUST 11, 2023
As effective anti-inflammatories , methylprednisolone, and its drug classmates can be used for various autoimmune , allergic, and other inflammatory conditions. Through a different mechanism, they can reduce inflammation effectively, particularly for musculoskeletal conditions.
The Checkup by Singlecare
NOVEMBER 16, 2023
You might also recommend vitamin D in patients at higher risk of a deficiency, including older adults or those with certain medical conditions like obesity and Crohn’s disease. A lack of vitamin D can result in thin, brittle, or misshapen bones, increasing the risk of fractures and conditions like osteoporosis and rickets in children.

The Checkup by Singlecare
MAY 11, 2023
The National Institute of Health defines women’s health as “the branch of medicine that focuses on the treatment and diagnosis of diseases and conditions that affect a woman’s physical and emotional well-being.” Hormone therapy: Some women may require hormone therapy to manage conditions such as menopause or infertility.
The Checkup by Singlecare
MARCH 4, 2024
Certain proton pump inhibitors Proton pump inhibitors (PPIs) are drugs used to treat various conditions, such as heartburn , gastroesophageal reflux disease (GERD), and certain types of ulcers. Alendronate Alendronate (Fosamax) is a bisphosphonate drug used to treat osteoporosis. Keep a list of medications and medical conditions.

BuzzRx
DECEMBER 14, 2022
The most common causes of bone fractures are trauma (sports injuries, motor vehicle accidents, falls), osteoporosis (a condition characterized by weak or brittle bones), and overuse or stress (this is more common in athletes). Fragility fractures are common in people with osteoporosis , a condition characterized by fragile bones.
The Checkup by Singlecare
AUGUST 14, 2023
Compare pantoprazole alternatives | Omeprazole | Esomeprazole | Lansoprazole | Dexlansoprazole | Rabeprazole | Natural alternatives | How to switch meds Pantoprazole is a commonly used prescription medication used to treat gastroesophageal reflux disease (GERD), peptic ulcer disease (PUD), and other stomach acid-related conditions.

Digital Pharmacist
JANUARY 16, 2023
Benefits of Creating a 2023 Health Awareness Calendar: Every month, national organizations and countries around the world come together to share educational content and promote services that support specific health conditions. National Osteoporosis Month. National Kidney Month. National Nutrition Month. National Stress Awareness Month.

European Pharmaceutical Review
SEPTEMBER 21, 2022
Teriparatide SUN (teriparatide) for the treatment of osteoporosis in adults. Converting the conditional marketing authorisations of the COVID-19 vaccines Comirnaty and Spikevax into standard marketing authorisations. Authorising the adapted bivalent vaccine Comirnaty Original/Omicron BA.4-5

The Checkup by Singlecare
JANUARY 9, 2024
Skin health Sea moss is used by some to reduce signs of aging and potentially treat dermatological conditions. While significant research has not been done, sea moss’s anti-inflammatory, antiviral, and antifungal properties could help treat rosacea, some acne, eczema, or other skin conditions.

The Checkup by Singlecare
FEBRUARY 14, 2024
Taking magnesium citrate may promote adequate magnesium levels in the body and prevent health conditions like osteoporosis.” Since hypertension can lead to serious medical conditions like cardiovascular disease and stroke, blood pressure regulation is important—and some studies have suggested magnesium citrate as a viable solution.
The Checkup by Singlecare
MAY 23, 2023
Dosages vary from one to three tablets a day, so for many conditions, 21 tablets will be anywhere from a seven-day supply to a 21-day supply. Depending on the condition being treated, there may be other lower-cost treatment options, including prescription or over-the-counter pain relievers such as ibuprofen.

The Checkup by Singlecare
AUGUST 9, 2023
They also support the gut microbiome, leading to improved overall health, and may prevent chronic health conditions like diabetes. Moderate yerba mate consumption is a healthy addition to your wellness arsenal if you don’t have health conditions that interact poorly with the tea.

BuzzRx
FEBRUARY 15, 2023
These distressing psychotic symptoms and sensory experiences can occur in various situations and medical conditions, such as alcohol and drug abuse, sleep deprivation, dementia, depression , and Charles Bonnet syndrome (a condition that causes people with vision impairment to have visual hallucinations).
The Checkup by Singlecare
DECEMBER 6, 2023
Because we offer up to 80% off, people know they can come to us to help them save on medications for sudden illnesses, chronic conditions, skin flare-ups, and more. The condition, which affects close to 5% of Americans , is controlled with medications like levothyroxine. Each year, SingleCare helps people save on prescriptions.

The Thyroid Pharmacist
FEBRUARY 17, 2023
Additionally, it can help asthma, osteoporosis, oxidative stress, chronic inflammation, and other concerns often seen in those with Hashimoto’s. 8] These two conditions are monumental drivers in almost every disease imaginable, including Hashimoto’s. It may also benefit other respiratory-related conditions. [21]

The People's Pharmacy
JUNE 19, 2023
Here is what the authors reported: Pulmonary Hypertension: This is a very serious health condition. The NIH describes it as: “…a condition that affects the blood vessels in the lungs. For example, can these drugs fight osteoporosis ? An article in the Annual Review of Pharmacology and Toxicology (Jan.

The Checkup by Singlecare
DECEMBER 1, 2023
Individuals with a higher risk of vitamin D deficiency or other medical conditions may need substantially more vitamin D to stabilize their levels. “ Vitamin D, often referred to as the ‘sunshine vitamin,’ plays a pivotal role in maintaining our overall health,” says Sherie Nelson MBA, RDN , Source Director of Wellness at Elior North America.

The Checkup by Singlecare
JANUARY 9, 2024
Low levels of magnesium may be linked to cramping and the condition Restless Leg Syndrome (RLS). Magnesium can also interfere with medications or existing health conditions. This form of magnesium is easily absorbed by the body and is less of a laxative than other forms of magnesium.

The Checkup by Singlecare
JANUARY 31, 2024
It’s used to treat many medical conditions, including asthma, certain types of cancer, rheumatoid arthritis, blood conditions, lupus, and immune system disorders. Osteoporosis Osteoporosis is a condition that makes your bones become weak and brittle.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content