How to Become a Medical Writer: A Comprehensive Guide
Viseven
AUGUST 7, 2024
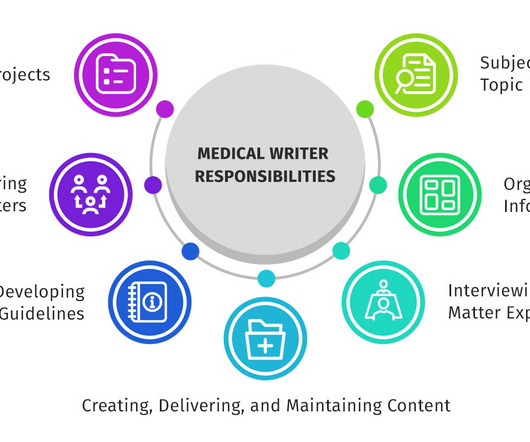
Medical writing refers to creating healthcare-related textual content that aims to improve doctor-patient communication and provide insights on industry topics. Medical writers must be outstanding communicators, capable of breaking down complex ideas for the public and healthcare professionals in a digestible way.





















Let's personalize your content