Rare Disease Day: Enhancing clinical trial success in rare diseases
pharmaphorum
FEBRUARY 28, 2024
Rare Disease Day is an opportunity to highlight the importance of enhancing clinical trial success in rare diseases for the benefit of patients.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

 clinical rare-diseases
clinical rare-diseases 
pharmaphorum
FEBRUARY 28, 2024
Rare Disease Day is an opportunity to highlight the importance of enhancing clinical trial success in rare diseases for the benefit of patients.

European Pharmaceutical Review
MARCH 28, 2024
Measuring treatment effect in rare disease populations presents many methodological challenges due to small sample sizes and heterogeneity of the study population. What are the key trends in clinical outcome assessments in clinical trials and what are the differences between those for common diseases and rare diseases?
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

pharmaphorum
FEBRUARY 27, 2024
Looking to the future of clinical trials: Gene therapy, precision medicine, and the ongoing quest for rare disease solutions Mike.Hammerton Tue, 27/02/2024 - 12:30 Bookmark this
Outsourcing Pharma
NOVEMBER 1, 2023
OSP had a quickfire interview with Rebecca Starkie, senior global patient engagement director, at Advanced Clinical where she explains the challenges faced by rare disease patients - and suggests ways to improve.

Pharmaceutical Technology
SEPTEMBER 18, 2023
Experts say a wider diverse pool of patients needs to be given a chance to participate in clinical trials to get rare disease drugs faster to market.

Fierce Pharma
JANUARY 2, 2023
Acasti, seeking to expand use of old steroid, posts clinical data on oral spray in rare disease. Tue, 01/03/2023 - 01:49.

Outsourcing Pharma
APRIL 5, 2021
The free Patient-Centric Trial Development Toolkit is aimed toward helping sites and sponsors identify and minimize risks in rare-disease clinical studies.

STAT
AUGUST 10, 2023
The serial dissembler of clinical trial results might be forced, finally, to tell the truth when it reads out its next study in Rett syndrome. What sets Anavex apart from all the other biotechs on my radar screen is its habit of shifting the goalposts on clinical trials. Anavex Life Sciences is in a tough spot.

STAT
FEBRUARY 28, 2023
Juvenile Batten disease — he has the type known as CLN3 — is stealing his childhood. And then this rare disease will steal my child. My toddler, Wheeler, will probably not live to adulthood. Wheeler is missing the DNA needed to recycle a waste product called lipofuscin that his cells naturally produce.
Fierce Pharma
FEBRUARY 15, 2024
Changing a clinical trial’s statistical analysis plan on the cusp of a readout? Changing a clinical trial’s statistical analysis plan on the cusp of a readout? That’s exactly what Alnylam just did for a closely watched study of its next-generation RNA interference therapy Amvuttra in a rare heart disease.
STAT
MARCH 28, 2024
The staff of the Institute for Clinical and Economic Review, or ICER, are known as the nerds of the drug industry: bespectacled killjoys who emerge a few times a year to scold drugmakers for pricing their latest cancer or MS advance far beyond reason. But last year, its staff sat down and concluded a forthcoming treatment was worth up to $3.9
STAT
FEBRUARY 15, 2024
A new report released Thursday by the Centers for Disease Control and Prevention appears to show that Lyme disease cases in the United States rose by 69% in 2022 over previous years. But in reality, the sharp increase is likely due to a new way of doing surveillance for the disease, not an explosion of cases.

Outsourcing Pharma
SEPTEMBER 20, 2021
Two experts from the contract research organization offer advice on planning and executing clinical studies with the unique needs of such patients in mind.

STAT
DECEMBER 8, 2022
Over the past few years, the biopharmaceutical industry has revved up efforts to diversify clinical trials. But clinical trials for rare diseases are still too often homogeneous. “We need to put this as a challenge to the CEOs and think about the environmental, social, and governance aspects of this as well.”
STAT
APRIL 15, 2024
Ultragenyx has found early success with its experimental treatment for the rare disease Angelman syndrome, supporting the company’s decision to acquire its development partner two years ago. The next step is a meeting with the Food and Drug Administration to discuss the findings and plans for a Phase 3 pivotal study.

Outsourcing Pharma
JULY 19, 2022
The CRO and medical genetics company will collaborate to accelerate trials centered on rare diseases, using real-world data and genetic testing technology.
European Pharmaceutical Review
APRIL 16, 2024
Novartis has released new data from the first and only Phase III study to demonstrate significant proteinuria reduction by targeting the complement system in patients with the rare kidney disease IgA nephropathy (IgAN). The APPLAUSE-IgAN clinical trial is ongoing and only limited interim analysis results can be presented.
European Pharmaceutical Review
FEBRUARY 29, 2024
billion , Biogen gained rights to SKYCLARYS ® (omaveloxolone), the first approved treatment for the rare disease Friedreich’s ataxia. These different options allow us to select the most appropriate modality based on the disease we are targeting.” Last year, in agreeing to acquire Reata Pharmaceuticals for $7.3

STAT
SEPTEMBER 29, 2023
In 2020, as biotech stocks surged amid the pandemic, a startup called Taysha Gene Therapies raised over $300 million off an audacious promise: It was going to license and develop gene therapies for at least 18 different rare and serious neurological diseases. Continue to STAT+ to read the full story…

STAT
DECEMBER 18, 2023
When faced with a loved one’s progressive neurodegenerative disease, like Alzheimer’s, or your child’s rare respiratory disease, you question why researchers and resource-backed pharma cannot bring a drug to market quickly enough to help your loved ones.
European Pharmaceutical Review
APRIL 5, 2024
Interim data from a Phase I/II clinical trial suggest that mRNA-3927, an investigational mRNA therapy from Moderna, could be a promising treatment for propionic acidaemia. I am particularly proud of these results given that there are currently no therapeutic treatments approved for patients with this disease,” Dr Holen explained.
Outsourcing Pharma
NOVEMBER 17, 2023
OSP was delighted to speak to Galina Nesterova, executive medical director at the Rare Disease & Pediatrics Center of Excellence, and Susan McCune, vice president of pediatrics and clinical pharmacology both within medical science and strategy at PPD, part of Thermo Fisher.

pharmaphorum
NOVEMBER 25, 2022
There are approximately 7,000 different types of rare disease and researchers estimate that there are more than 300 million people worldwide living with such a condition, according to the National Institutes of Health (NIH). A rare disease is any disease that affects a small percentage of the population.
pharmaphorum
JANUARY 19, 2021
A survey by rare disease patient network Raremark found that 86% of the community members asked were interested in taking part in clinical trials. CEO Jeremy Edwards looks at how decentralised trial models can solve some of the challenges for clinical trial recruitment in rare disease.

PQA
MARCH 8, 2024
February 29 was Rare Disease Day, providing an opportunity to raise awareness of the impact of rare diseases. Ensuring the quality of medication use is critically important for rare disease patients, payers, clinicians, specialty pharmacies and everyone with a role in the care process. Learn more below.
European Pharmaceutical Review
AUGUST 21, 2023
According to a market report , the global RNA therapy clinical trials market is anticipated to reach $3.5 This has therefore led to a rise in the number of RNA clinical trials being conducted. This has also resulted in the pharmaceutical industry investigating RNA as a target to treat other infectious diseases and oncology indications.
European Pharmaceutical Review
SEPTEMBER 15, 2023
The CBL-0201DD Phase II study evaluating the first-in-class small-molecule drug CBL-514 for Dercum’s disease, a rare disorder, has demonstrated significant efficacy in reducing lipoma size and complete clearance. Dercum’s disease is characterised by painful lipomas developing primarily on the trunk in the body.

pharmaphorum
DECEMBER 13, 2022
A new initiative to boost research and development into rare and paediatric diseases launched last week, on Thursday 8 th December, at the European Health Summit. These are diseases which currently have no therapeutic options and which, sadly, often affect the youngest patients. million rare disease patients.

European Pharmaceutical Review
NOVEMBER 27, 2023
Alexion’s (AstraZeneca Rare Disease) intravenous treatment is recommended for children who have been diagnosed with Wolman Disease at two years old or younger. This ultra-rare metabolic disease occurs due to lysosomal acid lipase (LAL) enzyme deficiency.

European Pharmaceutical Review
DECEMBER 19, 2023
Ionis will be responsible for non-clinical and clinical development of donidalorsen, Otsuka will apply for regulatory approval and commercialise the drug in Europe. “We HAE is a rare genetic disease characterised by unpredictable and frequently severe swelling of the skin, GI tract, upper respiratory system, face, and throat.

STAT
JUNE 27, 2023
But developing treatments for patients with rare diseases — conditions that afflict fewer than 200,000 people in the United States — is particularly challenging. When there are only a few thousand or even a few hundred people living with a particular condition, finding enough patients for clinical trials can take years.
STAT
FEBRUARY 7, 2023
Major advances in the study and treatment of rare diseases occurred in 2022, from a better understanding of just how many of these diseases exist to the start of new clinical trials that may lead to new therapies. According to the report, the earlier estimates failed to represent the full spectrum of these diseases.

European Pharmaceutical Review
OCTOBER 19, 2022
The US Food and Drug Administration (FDA) has awarded a total of $38 million in the form of 19 new grants and two new contracts, to facilitate the upcoming four years of clinical trials and regulatory tools for rare diseases. More than $25 million will aid 11 clinical trials to develop treatments for rare diseases.
European Pharmaceutical Review
APRIL 24, 2024
To treat this rare disease , GNT0004 contains a shortened, functional version of DMD gene (hMD1) encoding dystrophin, according to Genethon. Clinical trials for rare diseases The post AAV gene therapy demonstrates positive activity in Duchenne muscular dystrophy appeared first on European Pharmaceutical Review.

Pharmaceutical Technology
MAY 16, 2023
The Foundation for the National Institutes of Health (FNIH) has announced its plans to prioritise eight rare diseases to provide industry standards for manufacturing, preclinical testing and product analytical testing for gene therapy development. This will include pairing up indications with manufacturers amongst the BGTC’s partners.

pharmaphorum
FEBRUARY 28, 2022
With over 40% of all medicines in the pipeline aimed at rare diseases, the future looks positive for increasing the number of treatments available. There are approximately 7,000 different types of rare disease and researchers estimate that there are more than 300 million people worldwide living with such a condition.

pharmaphorum
JANUARY 25, 2021
Launch timelines have been delayed and clinical trials have been postponed or suspended. As a result, life sciences companies are turning to novel methods of collecting clinical data and innovative trial design. In cases of fatal diseases, recruiting a control arm is also considered unethical. DOWNLOAD THE FULL ARTICLE HERE.

STAT
JANUARY 30, 2023
Since it was signed into law in 1983, the FDA has approved more than 1,100 treatments for rare diseases. The act also created an industry that didn’t exist in the United States before its enactment, enabling the formation of companies to develop and commercialize therapies for rare diseases. Read the rest…

STAT
SEPTEMBER 7, 2022
Every time I read about clinical trials testing possible treatments for rare diseases, I think of my son, Ty, whose brief but successful foray into such a trial highlights their value and their devastating limitations. Read the rest…

European Pharmaceutical Review
DECEMBER 22, 2023
Alexandria Forbes, PhD, president and chief executive officer of MeiraGTx said the deal puts the company in a strong financial position and allows it to increase focus on two late-stage clinical programmes in Xerostomia and Parkinson’s disease and on its manufacturing capabilities. “In
European Pharmaceutical Review
MAY 8, 2023
A marketing authorisation for PRX-102 (pegunigalsidase alfa) , the first Poly(ethylene glycol) (PEG)ylated enzyme for Fabry disease, has been granted by the European Commission (EC). CHMP recommends first pegylated enzyme for Fabry disease… The PEGylated enzyme replacement therapy Chiesi Global Rare Diseases and Protalix BioTherapeutics, Inc.

European Pharmaceutical Review
SEPTEMBER 4, 2023
The National Institute for Health and Care Excellence (NICE) has recommended Chiesi’s Elfabrio ® (pegunigalsidase alfa) for Fabry disease (alpha-galactosidase deficiency) in adults. Data highlighted by Chiesi noted that the efficacy and safety profile of pegunigalsidase alfa was drawn from the outcomes of a clinical trials programme.
pharmaphorum
AUGUST 4, 2021
Spinal Muscular Atrophy (SMA) is a rare disease affecting the motor nerve cells in the spinal cord. This SMA Awareness month , we look at how digital health solutions can complement and enhance the effectiveness of drug-based treatments for rare diseases and make a positive difference in the life of patients.
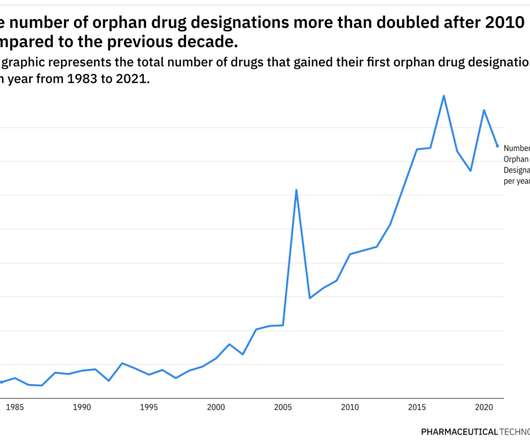
Pharmaceutical Technology
JUNE 29, 2022
This year has already been eventful when it comes to the development of therapies for rare diseases. Additionally, pricing and access for rare disease therapies continue to be scrutinized closely. Prior to the program, only 10 drugs were approved for a rare disease.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content