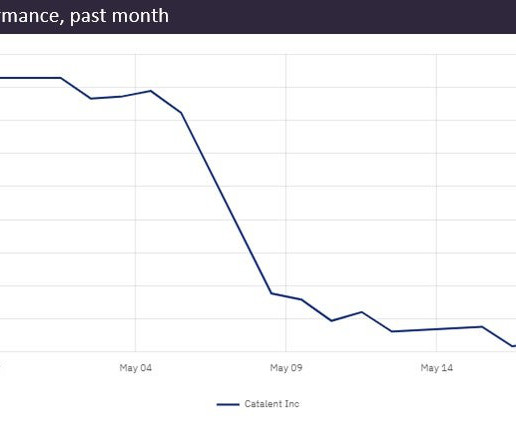
Catalent CEO drops FY2023 revenue forecast by $450m after “disappointing” quarter
Pharmaceutical Technology
MAY 30, 2023
In an investor call on 19 May, Catalent reduced its annual net revenue forecast by approximately 10% compared to its February predictions. Catalent has enough drug stock to meet immediate customer demand, he added. Source: GlobalData Pharmaceutical Intelligence Center (Accessed 24 May 2023). ©GlobalData.















Let's personalize your content