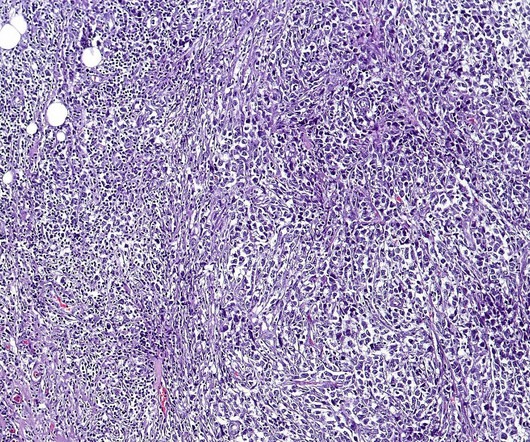
FDA approves AbbVie-Genmab’s Epkinly to treat DLBCL
Pharmaceutical Technology
MAY 22, 2023
“The FDA approval of Epkinly represents a new treatment mechanism of action for third-line DLBCL patients. “As As a non-chemotherapy, single-agent treatment for DLBCL patients, we hope that Epkinly can effectively treat this aggressive cancer type and can be used for patient care quickly and in an off-the-shelf form for physicians.







Let's personalize your content