Health Canada gives approval to Enhertu for breast cancer treatment
Pharmaceutical Technology
JANUARY 13, 2023
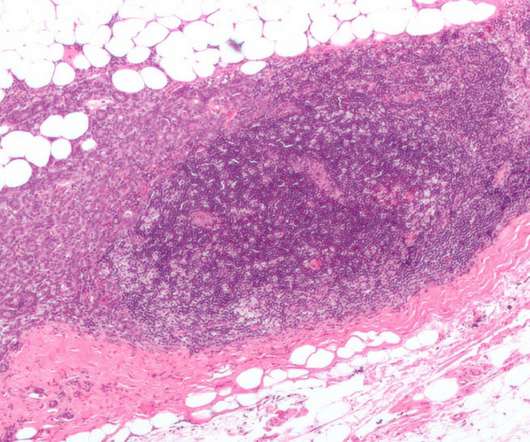
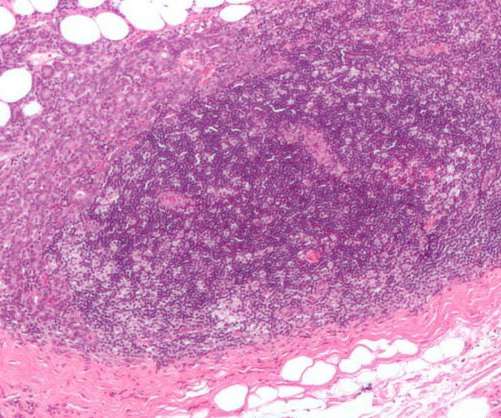
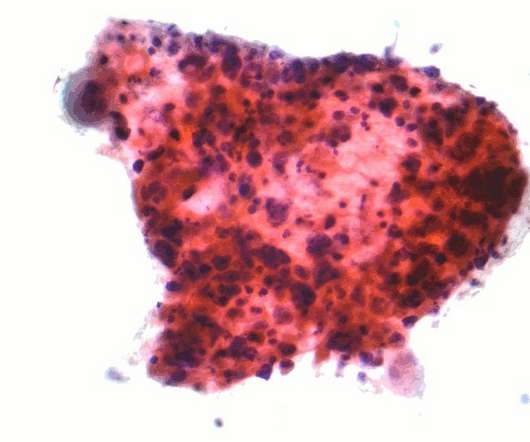
Enhertu has been approved to treat HER2-low breast cancer adult patients who have previously received at least one line of chemotherapy in the metastatic setting or who have seen disease recurrence during or within six months after the adjuvant chemotherapy. Enhertu’s safety profile was consistent with the previous clinical trials.



















Let's personalize your content