FDA Approves GSK's BLA for 5-in-1 Meningococcal Vaccine
Drug Topics
APRIL 16, 2024
The company’s investigational ABCWY vaccine candidate will be reviewed by the FDA by February 14, 2025.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.
Drug Topics
APRIL 16, 2024
The company’s investigational ABCWY vaccine candidate will be reviewed by the FDA by February 14, 2025.
STAT
MARCH 5, 2024
Experts who advise the Food and Drug Administration on vaccine-related issues voted unanimously on Tuesday to recommend that the FDA approve trivalent flu vaccines for the 2024-2025 season, instead of the quadrivalent, or four-in-one, shots that have been the industry standard for the past decade or so. Read the rest…
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Outsourcing Pharma
MARCH 23, 2021
A new report from the organization forecasts a wave of pharma innovation over the next four years could lead to 75 FDA drug approvals annually by 2025.

pharmaphorum
AUGUST 24, 2022
Amgen has reported positive phase 3 results with its biosimilar version of AstraZeneca/Alexion’s blockbuster rare disease drug Soliris, setting up a regulatory filing with the FDA. The post Amgen’s Soliris biosimilar clears phase 3, but won’t launch until 2025 appeared first on.

STAT
DECEMBER 21, 2023
But planning isn’t the same as doing, the industry’s track record isn’t great, and it’s not clear whether the FDA will twist arms, experts told STAT. Congress in late 2022 passed a law requiring companies to give FDA their plans for diversifying clinical trials.

Pharmacy Times
APRIL 16, 2024
The 5-in-1 meningococcal ABCWY vaccine candidate has an assigned Prescription Drug User Fee Act action date of February 14, 2025.

Fierce Pharma
NOVEMBER 1, 2023
The FDA has signed off on Amgen’s biosimilar version of Johnson & Johnson’s autoimmune standout Stelara. | The FDA has approved Amgen’s biosimilar version of Johnson & Johnson’s autoimmune standout Stelara.

The Checkup by Singlecare
NOVEMBER 6, 2023
Food and Drug Administration (FDA) just approved Wezlana (ustekinumab-auub), a biosimilar for the popular drug Stelara. According to the FDA announcement, the most serious side effect of Wezlana is infection because the prescription affects your immune response. How much will Wezlana cost?

Pharma Mirror
MARCH 10, 2021
The report anticipates breakthroughs in multi-modal disease therapies within five years, mRNA platforms to transform many new non-infectious disease treatment options, and huge growth in contract services due to the recent surge in IND applications – with 75 annual FDA approvals expected by 2025.

Fierce Pharma
OCTOBER 24, 2023
The FDA has accepted AZ’s supplemental biologics license application (sBLA) for self-administered FluMist Qudrivalent. The company expects a decision in the first quarter of next year and is making plans to launch the treatment in the 2024-2025 flu season.

IDStewardship
APRIL 14, 2024
Candidate 2025 Mentored by: Christina G. These advancements are evidenced by the two FDA-approved FMT products available for rCDI prevention: Rebyota (Fecal Microbiota, Live-jslm) and Vowst (Fecal Microbiota Spores, Live-brpk). Rebyota, a solution delivered via enema, was the first FMT product to be FDA-approved. Gut Microbes.

European Pharmaceutical Review
NOVEMBER 2, 2023
The US Food and Drug Administration ( FDA) has approved Wezlana (ustekinumab-auub) as a biosimilar to Johnson & Johnson’s Stelara (ustekinumab). Stelara biosimilars in the US market The first Stelara biosimilars are expected to enter the US market in 2025. “We US sales of Stelara totalled $6.4

pharmaphorum
MAY 18, 2022
FDA veteran Bakul Patel has joined Google as its new senior director of global digital health strategy, ending a stint at the regulator that lasted more than 13 years. Current FDA Commissioner Robert Califf spent some time as an advisor to Google parent Alphabet after his earlier stint at the head of the regulator. Bakul Patel.
Pharmaceutical Technology
OCTOBER 2, 2023
An NDA submission for EryDex is currently intended for Q4 2025, assuming positive Phase III study results.

Pharmacy Times
OCTOBER 18, 2023
Jensen notes that although she expects there will be a lot of FDA approvals in 2023 and 2024, there likely will not be another biosimilar “boom” until 2025.

The FDA Law Blog
OCTOBER 12, 2023
Koblitz — For years, submitting a Suitability Petition has been like screaming into a void: You’d be lucky if FDA ever responds. This has been a big problem because FDA’s inattentiveness can delay entry of certain types of ANDAs for years—often resulting in the ANDA applicant’s abandonment of the Suitability Petition.

STAT
SEPTEMBER 13, 2023
In connection with the move away from prescription sales, Akili will reduce its workforce by 40%, which the company projects will extend its runway into the second half of 2025. Akili went public last year and though its prescription sales were slowly increasing, the company’s costs far outstripped its revenues.

STAT
NOVEMBER 1, 2023
FDA staff said the new type of technology raised concerns about unintended genomic alterations that can potentially cause other side effects, but did not raise any concerns about efficacy. If the therapy is approved, Vertex has proposed a 15-year follow up of patients to evaluate the safety outcomes of the therapy. billion in 2022.

Fierce Pharma
NOVEMBER 30, 2023
22, 2025, pending an FDA approval. As Johnson & Johnson’s top moneymaker Stelara nears its patent cliff, biosimilar makers are eager to launch their own versions of the blockbuster immunology med. Samsung Bioepis' biosimilar, which Sandoz has agreed to commercialize, can launch on Feb.
pharmaphorum
DECEMBER 29, 2021
Sanofi and Regeneron have another challenger to their big-selling drug Dupixent for atopic dermatitis, now that the FDA has approved Leo Pharma’s rival antibody tralokinumab. The company has put tralokinumab at the heart of plans to significantly expand its product sales, R&D pipeline and global presence by 2025.

Pharmaceutical Technology
JUNE 1, 2023
Additionally, when asked whether the FDA should consider a potential accelerated approval, panellists voted 15 to one against that regulatory pathway for the drug. Additionally, when asked whether the FDA should consider a potential accelerated approval, panellists voted 15 to one against that regulatory pathway for the drug.

Pharmaceutical Technology
APRIL 21, 2023
On 14 April, the FDA rejected Lilly’s biologic licence application (BLA) for their anti-interleukin (IL)-23, mirikizumab, which is in development for the treatment of ulcerative colitis (UC). According to GlobalData’s patient-based forecast model, Stelara is anticipated to have US sales of $525.5m

The FDA Law Blog
OCTOBER 5, 2022
By Riëtte van Laack — On September 28, 2022, FDA announced the availability of the proposed rule for the implied nutrient content claim “healthy.” The term healthy, as an implied nutrient claim, was first defined by FDA in 1994. FDA also announced it would be re-evaluating the regulatory criteria for use of the “healthy” claim.

Pharmaceutical Technology
MAY 19, 2023
The US Food and Drug Administration (FDA) has approved AbbVie’s Rinvoq (upadacitinib) for patients with Crohn’s disease who do not respond to TNF blockers, a common immune suppressant treatment for the condition. The FDA nod means Rinvoq is now approved for seven indications in gastroenterology, rheumatology and dermatology.

The FDA Law Blog
MARCH 21, 2024
Now in the second year of implementation, companies have started noticing the consequences as FDA implements the new requirements and develops regulations and guidance. The MoCRA rollout, though, does not preclude that FDA may practice some regulation by enforcement if it deems such action necessary.

European Pharmaceutical Review
FEBRUARY 27, 2024
Novel biosimilar approval In the same month, the US Food and Drug Administration (FDA) approved Amgen’s Wezlana (ustekinumab-auub) as the first biosimilar to reference blockbuster drug Stelara (ustekinumab). The post Amgen opens its most advanced manufacturing facility to date appeared first on European Pharmaceutical Review.
STAT
SEPTEMBER 23, 2022
As I write this, there are 38 FDA-approved biosimilars ; 22 of them are commercially available. billion in 2020 (three times higher than savings from 2019) and have the potential to increase to $133 billion by 2025. In the U.S., the first biosimilar was launched in September 2015. Read the rest…

pharmaphorum
AUGUST 22, 2021
Bristol-Myers Squibb has scored a win in its drive to get Opdivo into earlier lines of cancer therapy, getting FDA approval for the drug for post-surgical treatment of invasive bladder cancer. ” The post BMS gets FDA nod for Opdivo as first adjuvant bladder cancer therapy appeared first on. .
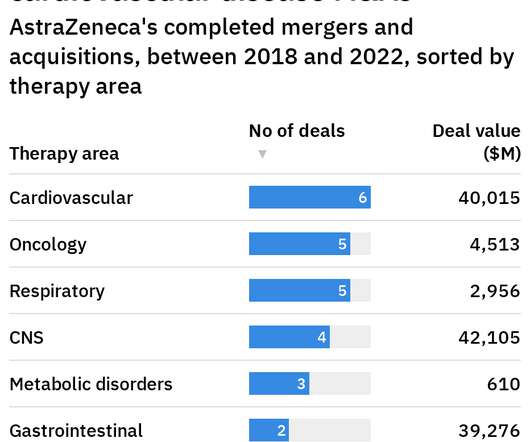
Pharmaceutical Technology
SEPTEMBER 5, 2022
Until 2025, we have strong growth ahead of us, but we also believe we can continue to grow very strongly post-2025 and it’s all about innovation in the pipeline.”. On 23 August, at a Reuters Newsmakers online forum, Soriot announced his interest in increasing AstraZeneca’s portfolio of bolt-on acquisitions leading up to 2025.

pharmaphorum
NOVEMBER 30, 2021
The FDA has kicked off its review of Bristol-Myers Squibb’s psoriasis therapy deucravacitinib, setting an action date of 1 September next year, as the EU and Japanese regulators also start their appraisals of the drug. The post FDA sets Sept review date for BMS’ psoriasis drug deucravacitinib appeared first on.

pharmaphorum
DECEMBER 22, 2022
The Parma site is due to become operational from 2024, with FDA, IFA, and EMA approvals due to be sought for 2025. In brief, the European plan for Chiesi is in-house drug development of biologicals and rare disease targeting.

The FDA Law Blog
OCTOBER 24, 2023
Mullen — As of October 1, 2023, all 510(k) submissions, unless exempted, must be submitted to FDA using the electronic Submission Template And Resource ( eSTAR ). Currently, eSTAR is voluntary for medical device De Novo submissions, but FDA has initiated the process of requiring De Novos to be submitted using eSTAR.

Pharmaceutical Technology
JUNE 7, 2023
Mounjaro’s approval for obesity, particularly in the US and EU markets, means the drug is entering a significantly large market where it will have direct competition from Novo Nordisk’s Wegovy (semaglutide), which is currently the leading glucagon-like peptide-1 (GLP-1) therapy approved for obesity by both the FDA and EMA.

The FDA Law Blog
OCTOBER 24, 2022
355g(a), directed FDA to “establish a program to evaluate the potential use of real world evidence” both “to help to support the approval of a new indication” and “to help to support or satisfy postapproval study requirements.” The sponsor and FDA reach agreement on the study design information to be publicly disclosed.

PharmaShots
FEBRUARY 19, 2023
Pfizer expects to submit a BLA to the US FDA & an MAA to the EMA for VLA15 by 2025 Ref: Globenewswire | Image: Valneva Related News:- Valneva and Pfizer Report Results of VLA15 in P-II (VLA15-221) Trial for the Treatment of Lyme Disease

pharmaphorum
JUNE 6, 2022
The results have set up a pre-filing meeting with the FDA, according to BMS. Rozlytrek was approved by the FDA in 2019 but has just started to gather sales momentum, making around $17 million in the first quarter of this year, an increase of 78% on the first three months of 2021.
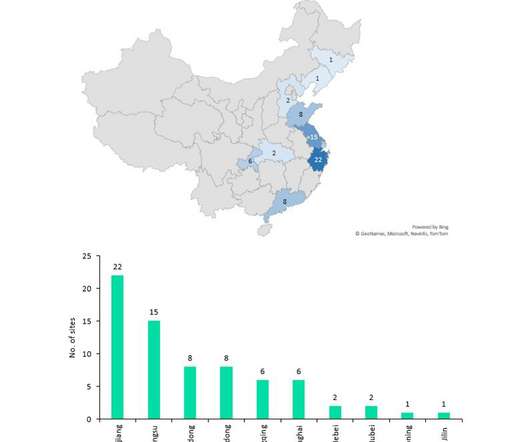
Pharmaceutical Technology
NOVEMBER 22, 2022
The Chinese government announced its ten-year 'Made in China 2025' strategic plan in 2015, which aimed to make the country a global leader in several high-tech industries. Guangdong is based in the south and has a comparatively strong number of manufacturing relationships producing innovative FDA and EMA drugs.

Pharmaceutical Technology
JANUARY 19, 2023
However, it is possible to target CD33 in the clinic, as evidenced by the FDA approval of Pfizer’s anti-CD33 antibody-drug conjugate Mylotarg (gemtuzumab ozogamicin) in 2000. NiCord’s biologics license application is currently being investigated by the FDA with a review date set by May 2023.

pharmaphorum
DECEMBER 14, 2022
Sodium oxybate — which in the late 1980s was marketed to bodybuilders and then became known as GHB and criminally used as a date rape drug — has been sold under the brand name Xyrem after gaining FDA approval in 2002. In 2020, the FDA-approved indication was expanded to include those patients who suffer from cataplexy.
pharmaphorum
SEPTEMBER 21, 2022
The European Commission has followed the lead of the US FDA and approved AstraZeneca’s Tezspire as an add-on maintenance therapy for patients with severe asthma, becoming the first and only biologic that can be used in all patients, and not restricted to those with specific forms of the disease. billion or more.

pharmaphorum
DECEMBER 6, 2021
billion, which should be enough to cover its cash burn through 2025, according to the company. The company’s AI platform also identified Eli Lilly’s JAK inhibitor Olumiant (baricitinib) as treatment for COVID-10, which now has emergency use authorisation from the FDA and is under review by the EU drugs regulator.

European Pharmaceutical Review
MARCH 6, 2023
The Moderna Innovation and Technology Centre is expected to become operational in 2025. Alongside development of this facility, Moderna continues to advance its mRNA portfolio.

pharmaphorum
APRIL 6, 2022
billion in its R&D last year as it pursues a goal of bringing 15 new products to market by 2025, the highest spend in its 137-year history and one which rivals some much larger big pharma groups. The German group has just reported a 28% increase in Jardiance (empagliflozin) sales to €3.9

pharmaphorum
OCTOBER 26, 2022
The drug was highlighted at Boehringer’s R&D update earlier this year as one of the most promising candidates among 15 new medicines it plans to bring to market by 2025, fuelled by a €25 billion R&D spend.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content