ESMO 2022: neoadjuvant or adjuvant immunotherapy for locally advanced cancers?
Pharmaceutical Technology
SEPTEMBER 13, 2022
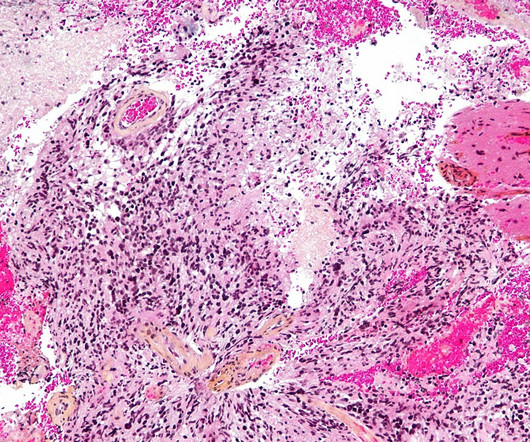
The advent of immune checkpoint inhibition has arguably been the greatest breakthrough for the treatment of metastatic solid tumours, with durable complete responses observed across multiple cancer types. month progression-free survival (PFS) benefit over SOC chemotherapy. MSI-H/dMMR CRC accounts for 10-15% of all CRC.


























Let's personalize your content