FDA-Approved Labeling: Is Enough Enough?
The FDA Law Blog
AUGUST 21, 2023
Livornese — I saw the sign…and the answer is no—FDA-approved labeling apparently is not enough under state failure-to-warn laws, according to certain courts.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

The FDA Law Blog
AUGUST 21, 2023
Livornese — I saw the sign…and the answer is no—FDA-approved labeling apparently is not enough under state failure-to-warn laws, according to certain courts.

Fierce Pharma
AUGUST 22, 2023
With a new FDA approval to rival Teva's Austedo, Neurocrine Biosciences’ Ingrezza is debuting in a treatment area with hundreds of millions of dollars of revenue potential. | The FDA approved Ingrezza capsules to treat adult with chorea associated with Huntington’s disease.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

The FDA Law Blog
DECEMBER 15, 2022
By Riëtte van Laack — On December 7, 2022, FDA announced the availability of the final guidance regarding the enforcement policy for homeopathic drug products. This concludes FDA’s reevaluation of the regulation of homeopathic drugs which it started in 2015. It issued a draft guidance in 2017 which was subsequently revised in 2019.

The FDA Law Blog
MARCH 5, 2024
Many individual states have pursued some type of legislation to restrict the use of traditional meat terminology for the labeling of APPs. Many states have proposed but failed to enact legislation regulating the labeling of APPs, in some cases due to concerns of potential legal challenges based on federal preemption claims.

The FDA Law Blog
MAY 23, 2023
We previously noted that “ the skinny label may be dead ” and, while we still can’t be sure if it’s truly gone (but not forgotten), we now know that the Supreme Court won’t hear this case at this time. Further, FDA, in its ministerial role, only looks at the use code and approves a carve-out based on the use code.
European Pharmaceutical Review
APRIL 8, 2024
The first BCMA-targeted CAR-T cell therapy for second-line treatment of multiple myeloma has been approved by the US Food and Drug Administration (FDA). Janssen), a Johnson & Johnson company in December 2017. The partnership deal focused on the development and commercialisation of the immunotherapy CAR-T cell therapy cilta-cel.

pharmaphorum
NOVEMBER 1, 2021
Novartis has claimed FDA approval for one of its ‘wild card’ drugs – Scemblix – a first-in-class STAMP inhibitor for patients with previously-treated chronic myeloid leukaemia (CML). The post Novartis’ ‘wild card’ drug Scemblix gets FDA nod for leukaemia appeared first on.
pharmaphorum
SEPTEMBER 30, 2022
Patient organisations have been celebrating the FDA approval yesterday of Amylyx’ amyotrophic lateral sclerosis (ALS) therapy Relyvrio, after not one but two advisory committee meetings that arrived at different conclusions about the drug. The post Relief for Amylyx as FDA clears controversial ALS drug Relyvrio appeared first on.
pharmaphorum
DECEMBER 18, 2020
GlaxoSmithKline’s Benlysta has been on the market for almost a decade, but it still has some tricks up its sleeve – it’s just become the first and only FDA-approved treatment for lupus nephritis. The post GSK’s Benlysta claims first FDA okay for lupus kidney damage appeared first on.
pharmaphorum
DECEMBER 29, 2021
Sanofi and Regeneron have another challenger to their big-selling drug Dupixent for atopic dermatitis, now that the FDA has approved Leo Pharma’s rival antibody tralokinumab. Dupixent’s label currently calls for dosing every two weeks for all patients. Leo now says it will start rolling the new product out in February.

pharmaphorum
FEBRUARY 8, 2021
Bristol-Myers Squibb finally has FDA approval for its CAR-T therapy liso-cel, which has been cleared by the US regulator as Breyanzi for certain forms of large B-cell lymphoma. DLBCL is the most common type of NHL in adults, accounting for around a third of the 77,000 new cases diagnosed in the US, according to the FDA.

pharmaphorum
JULY 8, 2021
The FDA has started a priority review of AstraZeneca and Amgen’s severe asthma antibody tezepelumab, setting a date for a decision in the first quarter of 2022. Tezepelumab was awarded a breakthrough designation for non-eosinophilic asthma by the FDA in 2018. Results from that programme are due in 2023.

STAT
JUNE 29, 2023
He allegedly worked with several other individuals and pharmacies between 2017 and 2021 as part of the scheme. Reconfiguring bottles and labels for distributing medicines is known as misbranding or diverting legitimate prescription drugs. Steven Diamantstein, who runs Scripts Wholesale in Brooklyn, N.Y.,

pharmaphorum
JUNE 7, 2021
Kymriah (tisagenlecleucel) was approved for relapsed/refractory ALL in 2017, but its label covers use in children and young adults aged up to 25, who account for the bulk of cases of the blood cancer. It was filed for the adult ALL indication in the US in April, and has a priority review from the FDA, with a verdict due in October.
The Checkup by Singlecare
FEBRUARY 19, 2024
Food & Drug Administration (FDA) for weight management, many people find they lose weight with these medications, especially with lifestyle changes like a healthy diet and regular exercise. Rybelsus is the first oral form of semaglutide, approved in 2017. This indication is not found on the label for Rybelsus.
The Checkup by Singlecare
DECEMBER 21, 2023
But since its FDA approval for the treatment of Type 2 diabetes back in 1994, metformin has emerged as something of a “miracle drug” thanks to its effectiveness in treating other common health conditions, like infertility, obesity , and heart disease. Metformin is a medication commonly used to treat people with Type 2 diabetes.
The Checkup by Singlecare
OCTOBER 12, 2023
Abilify generic | Abilify vs. aripiprazole | Cost | Off-label use | How to switch Abilify is a brand-name medication approved to treat certain mental health conditions, including the treatment for schizophrenia , bipolar disorder , and major depressive disorder. Abilify first received FDA approval in 2002. mL, 960 mg/3.2

The FDA Law Blog
SEPTEMBER 28, 2023
Walsh — As readers of the FDA Law Blog know, the FDC Act is a strict liability criminal enforcement statute that can impose criminal misdemeanor penalties on a person without any showing of intent. Richard Marschall, a “naturopathic doctor,” had been convicted in 2017 for a misdemeanor violation of the FDC Act for selling misbranded drugs.

The Checkup by Singlecare
JANUARY 26, 2024
Approved by the Food and Drug Administration (FDA) in 2017 for blood sugar management, Ozempic has recently gained attention for its weight loss effects. Healthcare professionals prescribe Ozempic off-label for weight management for patients with obesity. It received FDA approval for weight management in June 2021.)

The Checkup by Singlecare
DECEMBER 28, 2023
Although Ozempic has been approved by the Food and Drug Administration (FDA) since 2017, its status as a household name is relatively recent. Weight loss is an off-label , non-FDA-approved use for Ozempic. The FDA has urged consumers to use caution when taking medication from these compound pharmacies.

pharmaphorum
MARCH 1, 2021
European regulators have refused to back GlaxoSmithKline’s daily triple therapy Trelegy Ellipta for asthma, denying a label extension because there was no evidence to show a reduction of flare-ups. But it fell short on the secondary endpoint of an improvement of a reduction in the annual rate of moderate to severe exacerabations.

PQA
DECEMBER 7, 2023
Certain classes, particularly glucagon-like peptide-1 (GLP-1) receptor agonists and the closely related glucose-dependent insulinotropic peptide-1/glucagon-like peptide (GIP/GLP-1) receptor agonists, have recently received US Food and Drug Administration (FDA) indications for weight loss in addition to diabetes treatment.

The Checkup by Singlecare
OCTOBER 30, 2023
Food and Drug Administration (FDA) to treat a wide range of respiratory bacterial infections, such as pneumonia and bronchitis , as well as infections of the ear , nose, throat , urinary tract infections , and skin. Amoxicillin food interactions According to the amoxicillin product labeling , there are no drug-food interactions of concern.
pharmaphorum
NOVEMBER 14, 2022
GSK has had another setback in its oncology business, after the FDA asked it to restrict use of its PARP inhibitor Zejula in ovarian, fallopian tube, or primary peritoneal cancer to patients with a specific mutation. That was due last Friday, but cancelled when GSK opted to withdraw the approval.

pharmaphorum
JANUARY 5, 2023
The deal centres on reSET and reSET-O, Pear’s cognitive behavioural therapy (CBT) based programmes, for people living with substance and opioid use disorders, respectively, which have been approved by the FDA as an adjunct to outpatient treatment.

PharmaShots
APRIL 20, 2023
Food and Drug Administration (FDA) in June 2020 as a rapid-acting insulin to improve glycemic control in adults with type 1 and type 2 diabetes. On October 14, 2022, the FDA approved Lyumjev® (insulin lispro-aabc injection), a rapid-acting mealtime insulin, for the treatment of children with diabetes.

Pharmaceutical Technology
JANUARY 23, 2023
Therefore, with an inadequate or loss of response to these anti-TNFs, clinicians must prescribe adult therapies off-label to treat younger patients. In addition to Skyrizi’s recent supplementary CD indication, both Tremfya and Skyrizi received US FDA approval in psoriasis in 2017 and in 2019, respectively.

pharmaphorum
OCTOBER 14, 2021
The findings were based on an ALITHIOS Phase 3b open-label extension study of Kesimpta, a targeted B-cell therapy that Novartis says, “delivers superior efficacy with a similar safety and tolerability profile compared with teriflunomide, a first-line treatment for MS.”. years vs initiation two years later. billion in 2019.
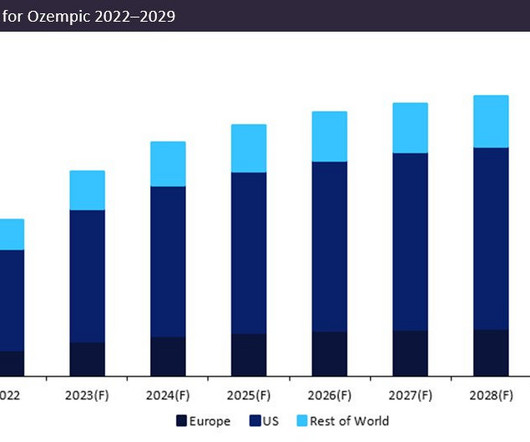
Pharmaceutical Technology
APRIL 28, 2023
Originally granted approval in its largest market, the US, in 2017, Ozempic has subsequently obtained approval for three distinct dosages, 0.5mg, 1.0mg, and 2.0mg, for the treatment of type 2 diabetes.

pharmaphorum
SEPTEMBER 21, 2020
The committee also recommended Lynparza should get its label extended to cover first-line maintenance treatment of ovarian cancer in combination with Roche’s Avastin (bevacizumab), in patients with homologous recombination deficient (HRD)-positive disease.
pharmaphorum
MARCH 8, 2021
The new indication for the Merck & Co/MSD-partnered drug – which is also used to treat ovarian, breast and pancreatic cancer – was approved by the EMA last year with a slightly more restricted label than was previous cleared by the FDA.
The People's Pharmacy
MARCH 20, 2023
Over the past several years, the FDA has approved a number of injectable medications designed to help people with type 2 diabetes control their blood sugar. The FDA approved Ozempic for use in the US only in 2017, so we have about five years of experience on long-term effects. That has led to drug shortages.

The Checkup by Singlecare
JANUARY 23, 2024
There are no medications with a Food and Drug Administration (FDA) approved indication for muscle relaxation available over the counter in the United States. There is one OTC drug product that is sometimes used off-label (without FDA approval) as a muscle relaxant, and that is guaifenesin. Dantrolene acts directly on the muscle.
The Checkup by Singlecare
APRIL 3, 2024
According to a 2017 study , the beverage acts in an anti-inflammatory manner on different components of the immune system, such as natural killer (NK) cells, monocytes, and neutrophils. This can reduce stomach upset unless your medication is labeled “enteric coated” or “gastro-resistant.” Take it in the morning.

pharmaphorum
SEPTEMBER 11, 2020
Ocrevus (ocrelizumab) was approved in 2017 for relapsing and primary progressive forms of MS and is seen as one of the most successful drug launches in pharma history, generating sales of $3.79 billion in 2019 with more growth predicted this year.
pharmaphorum
MARCH 9, 2022
The launch of Hemlibra (emicizumab) in 2017, with a label expansion in 2018 to broaden its use – had a speedy impact on Eloctate, which also came from Bioverativ’s stable. Sanofi recorded a $2 billion charge in 2019 that it said was largely down to pressure on its drug.

The Checkup by Singlecare
JANUARY 18, 2024
Drug interactions | Food interactions | Other interactions | Avoiding interactions | When to see a doctor Ozempic (semaglutide) has been the talk of the nation, more so for its use for weight loss rather than its actual Food and Drug Administration (FDA) indication, type 2 diabetes mellitus.

The Checkup by Singlecare
FEBRUARY 13, 2023
According to the FDA , eating a balanced diet high in magnesium may reduce your risk of hypertension, but the evidence supporting that claim is still inconclusive. Read the labels. Because the FDA doesn’t regulate supplements for effectiveness or safety, some third-party organizations have taken on that job.

pharmaphorum
APRIL 30, 2021
The FDA’s Oncologic Drugs Advisory Committee (ODAC) has voted to strip Merck & Co’s Keytruda of its accelerated approval in gastric and gastroesophageal junction (GEJ) cancer, despite a lack of treatment options in these patients. .
The Checkup by Singlecare
DECEMBER 5, 2023
Levothyroxine (a thyroid hormone) is FDA approved to treat hypothyroidism , a condition in which the body produces less thyroid hormone than your body needs. It’s FDA approved to treat attention-deficit/hyperactivity disorder (ADHD) symptoms, including inattention, hyperactivity, and impulsivity. Approximately 1.2%
The People's Pharmacy
JUNE 26, 2023
When she died, she had just been put on an antidepressant with a black-box FDA suicide warning. We had many conversations with representatives from Lilly and the FDA about our concerns regarding Prozac and suicidal thoughts. 11, 2017) discusses the connection between antidepressants and suicide.

The Checkup by Singlecare
APRIL 15, 2024
Anxiety Unlike depression, anxiety is an off-label use for trazodone. Insomnia Trazodone for insomnia is another off-label use, but a fairly common one. A systematic review from 2017 analyzed various studies where patients taking trazodone for insomnia reported significant improvements.

pharmaphorum
SEPTEMBER 25, 2020
The drug is approved both as a monotherapy in first-line NSCLC and also got the green light for use in combination with chemo in 2017. Also in May, Roche’s PD-L1 inhibitor Tecentriq (atezolizumab) claimed approval from the US regulator as a first-line monotherapy for NSCLC with 50% or greater PD-L1 expression.

pharmaphorum
FEBRUARY 2, 2021
Gocovri also demonstrated sustained efficacy for at least two years in the phase 3, open-label EASE LID-2 study, the company added. Neurocrine had to delay the launch of Ongentys until September after an FDA approval in April because of disruption caused by COVID-19.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content