Gilead’s Vemlidy expands label to treat paediatric chronic HBV
Pharmaceutical Technology
MARCH 29, 2024
Vemlidy was first approved to treat adults with HBV in 2016. Its label was expanded in 2022 for use in patients 12 years and older.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

Pharmaceutical Technology
MARCH 29, 2024
Vemlidy was first approved to treat adults with HBV in 2016. Its label was expanded in 2022 for use in patients 12 years and older.

The FDA Law Blog
APRIL 21, 2024
Accordingly, had Taiho marketed the product with labeling containing those errors, that labeling would have been false. Thus, regardless of the technicality of an initial notification letter stating approval on September 30, 2022, Taiho was prohibited under the FDCA from marketing LYTGOBI with false labeling, 21 U.S.C.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Psychadelic Pharmacist
JULY 26, 2021
In no particular order: Psilocybin with Psychological Support for Treatment-Resistant Depression Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study (2016).
pharmaphorum
JULY 1, 2021
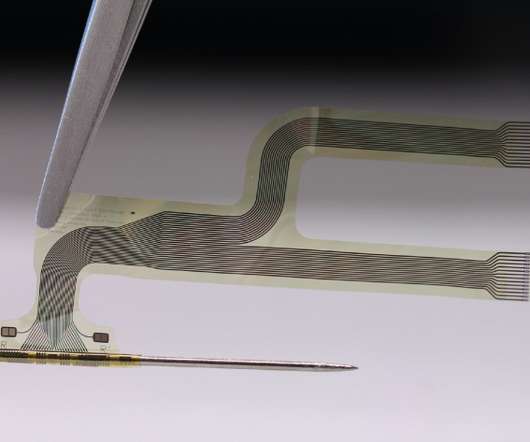
neuroloop – a spinout of Freiburg University in Germany formed in 2016 – has been working to date on using its device as an implant to lower blood pressure, among other applications. The Merck collaboration will concentrate on using the device alongside anti-inflammatory drug therapies.

pharmaphorum
AUGUST 23, 2022
The phase 1/2 trial will be an open-label, dose-escalation study that will test various doses of BV-101 in between 12 and 18 subjects. In 2019, Bayer bought cell therapy company BlueRock Therapeutics, which was created in 2016 via a joint venture between Bayer and Versant Ventures.

The FDA Law Blog
OCTOBER 5, 2022
In 2015, FDA issued a Warning Letter to Kind LLC because, among other things, the company labeled products as healthy whereas these products provided more saturated fat per serving than permitted by the regulatory definition. It is focused on the nutrients in the food product rather than on it overall nutritional “quality.”.
pharmaphorum
JUNE 8, 2022
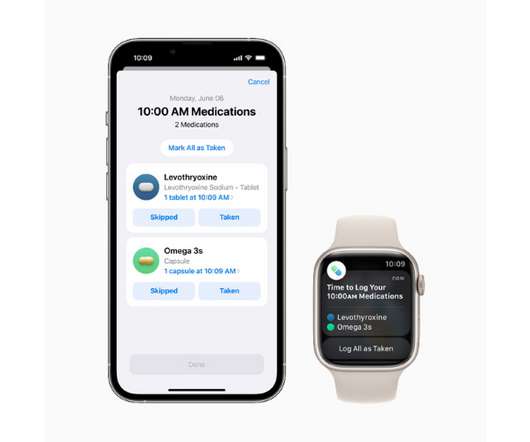
The new tool works as a component of Apple’s Health app and will let users add drugs or other health products like vitamins and supplements to a personal list – either by scanning a label or finding the product in a directory – and create custom schedules for them.
The Checkup by Singlecare
DECEMBER 21, 2023
Though metformin isn’t technically approved for use in treating anything other than diabetes, many healthcare providers prescribe it off-label because of the growing body of evidence supporting these added benefits. What is metformin approved to treat?

The Checkup by Singlecare
JANUARY 4, 2024
In addition, the FDA label for the nighttime forms of Mucinex containing acetaminophen recommends avoiding three or more drinks per day. Read all medication labels and consult a healthcare provider for medical advice before consuming alcohol while taking medications for cough and cold symptoms.

pharmaphorum
NOVEMBER 15, 2021
Updated labelling for Invokana has already been approved to include data showing that it can reduce the risk of hospitalisation for heart failure and diabetic kidney disease in patients with type 2 diabetes, based on the CREDENCE trial. . billion in 2016 before the product was linked to an increased risk of lower limb amputation.

pharmaphorum
JUNE 6, 2022
Xalkori is billed as an ALK inhibitor and has been approved to treat ROS1-positive NSCLC since 2016, with sales of $22 million in the first quarter, but is also used to treat ALK-mutated NSCLC and lymphoma. billion product, mainly from first-line use, if it can claim FDA approval for a broad label covering any ROS1-positive cancer.

The People's Pharmacy
MARCH 20, 2023
I also check labels for sodium and stay away from it as much as possible. Most agree that beets’ high level of nitrate contributes to nitric oxide in the blood vessels ( d’El-Rei et al, International Journal of Hypertension , online March 21, 2016 ; Omar et al, Journal of Internal Medicin e, April 2016 ).

The Checkup by Singlecare
DECEMBER 1, 2023
Red Dye 40 is among the most commonly used artificial food dyes in the United States, and you might see it labeled under a different name on the products you consume. Regardless of what manufacturers label it as, there have been ties to specific negative side effects when ingested too frequently. 40 Aluminum Lake, and FD&C Red No.

pharmaphorum
FEBRUARY 23, 2022
The drug was originally rejected for schizophrenia and bipolar by the FDA back in 2013 with a request for more clinical data, finally getting a green light two years later, and it then failed a phase 3 study in depression in 2016. Vraylar is now one of AbbVie’s fastest-growing products with sales expected to reach around $2.2

The Checkup by Singlecare
FEBRUARY 8, 2024
Clopidogrel and omega-3 fatty acids Omega-3 fatty acids are a popular dietary supplement, often labeled as fish oil capsules. Before you get too upset about this, check with your healthcare provider to see if any change in your coffee consumption is actually advised. Little known to most people, omega-3 fatty acids inhibit platelet activity.
The Checkup by Singlecare
SEPTEMBER 14, 2023
Based on clinical trials, the generic option was first approved in 2016, 13 years after AstraZeneca received the green light for the brand-name Crestor from the U.S. Off-label use of the drug is limited from its official indications as outlined by the FDA. Food and Drug Administration (FDA).

pharmaphorum
AUGUST 11, 2022
These drugs have been rigorously tested by regulatory bodies around the world before they’re made available to ensure they work as labelled, but despite that, adverse events crop up. In 2016, the estimated annual cost of drug-related morbidity and mortality resulting from non-optimised medication therapy was $528.4

The FDA Law Blog
SEPTEMBER 6, 2022
This Draft Guidance, when finalized, will replace the Final Guidance issued just six years ago (the 2016 Guidance). Changes from the 2016 Guidance. Livornese — FDA recently published a Draft Guidance entitled “Charging for Investigational Drugs under an Investigational New Drug Application: Questions and Answers” (the Draft Guidance).
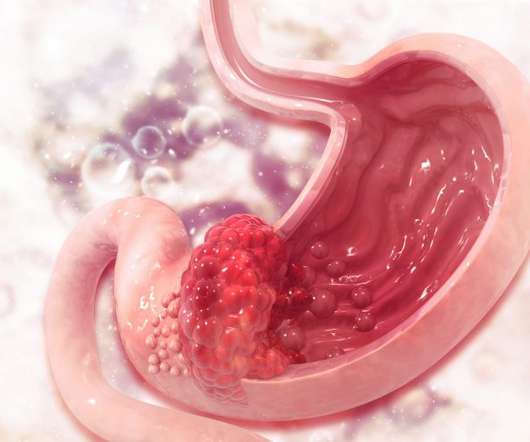
European Pharmaceutical Review
JULY 5, 2022
In the future, the potential label expansion of Oncoral into other solid cancer indications will be investigated. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up, Gastrointestinal Cancers , 2016; 27(suppl. accessdata.fda.gov/drugsatfda_docs/label/2014/020571s048lbl.pdf [Accessed 25.01.22].
The Checkup by Singlecare
MARCH 21, 2024
Jardiance was initially approved to treat Type 2 diabetes in 2014 but has achieved the following additional FDA approvals in the years since: In 2016, it was approved to reduce the risk of cardiovascular death in adults with Type 2 diabetes. But unlike the other drugs, Jardiance has evidence to support its different uses.
pharmaphorum
JULY 12, 2021
Merck’s chief science and technology officer Laura Matz said that Innerva’s platform could become “a true enabler for digital personalised treatment of patients suffering from severe and chronic diseases such as inflammatory disorders” An open-label, proof-of-concept study looking at a vagus nerve-stimulating implant as a therapy (..)

pharmaphorum
SEPTEMBER 25, 2020
The new data – presented at this year’s ESMO meeting – reinforce the massive improvement in patient care that Keytruda has achieved in previously-untreated NSCLC, which had a five-year survival rate of just 5% before the drug was approved in 2016.

The FDA Law Blog
APRIL 25, 2023
Note that section 3060(a) of the 21st Century Cures Act in 2016 amended section 520 of the FD&C Act and removed certain software functions from the statutory definition of a medical device. It applies whether the software is the entire device (i.e., Software in a Medical Device, or SiMD). Loose Ends IDEs.

pharmaphorum
JUNE 20, 2022
The serotonin (5-HT2A) receptor-selective inverse agonist has been approved for Parkinson’s psychosis since 2016, and brought in $481 million from that indication last year, but remains Acadia’s only commercial product. At one point it had been tipped as a future blockbuster.

The FDA Law Blog
NOVEMBER 29, 2022
Houck — On November 4th, CDC issued its revised guideline on prescribing opioids for pain as an expansion and update of its 2016 CDC Opioid Prescribing Guideline. We blogged on the 2016 guideline here in March 2016, and the proposed guideline here on March 18th). By Larry K. Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R.,

pharmaphorum
OCTOBER 27, 2020
The PolarisDMD study failed across the board, leaving Catabasis with no choice but to abandon the drug, including an ongoing open-label extension study from an earlier failed trial in the muscle-wasting disease. We are deeply saddened and disappointed by the results of our Phase 3 PolarisDMD trial,” said Jill Milne, Catabasis’ CEO. “I

The Checkup by Singlecare
FEBRUARY 1, 2023
According to a 2016 study, 97 patients with mild hyperlipidemia (high cholesterol) were treated with 300 mg of berberine capsules for three months. Always read the label. These organizations offer quality testing that shows a supplement contains only the labeled ingredients and doesn’t pose any potential risk. Pharmacopeia.

pharmaphorum
OCTOBER 27, 2022
The two companies have been working together on this microbiome project since 2016. In the open-label extension study ECOSPOR IV (SERES-013), 263 adults with rCDI were evaluated when given a commercial dose of SER-109 to fulfil FDA requirements for the oral therapeutic’s safety database.

Druggist
SEPTEMBER 15, 2020
Zovirax cream is available in two different pack types, each containing 2g of the cream: Aluminium tube Pump pack Zovirax Cold Sore Cream Aciclovir Tube 2 g Zovirax contains aciclovir, an anti-viral treatment; always read the label Fights the cold sore virus Works at the tingle and blister £6.98 Buy on Amazon Price incl.

The Checkup by Singlecare
JANUARY 18, 2024
If symptoms of an interaction develop, such as abdominal pain, hypoglycemia, or blurred vision, the matter takes on a sense of urgency.

pharmaphorum
JULY 11, 2021
The letter follows the extraordinary situation in which Biogen and partner Eisai asked the FDA to restrict the label for Aduhelm, as the agency approved the drug for all Alzheimer’s patients, not the mildly-affected population tested in clinical trials. pic.twitter.com/iWJNxdZ5Cs.

The Checkup by Singlecare
DECEMBER 26, 2023
Instead of assuming manufacturers are selling what they’re promising on the supplement label, experts suggest checking with a third-party organization that tests supplements, such as Consumer Lab , NSF International , or U.S. In fact, the FDA does not review dietary supplements before they’re sold to customers. Pharmacopeia.
The Checkup by Singlecare
DECEMBER 12, 2023
Sources Zolpidem: drug information , UpToDate (2023) Ambien label , FDA (2014) Ambien drug label , NIH DailyMed (2019) New safety measures announced for opioid analgesics, prescription opioid cough medication, and benzodiazepines , FDA (2016) The post Zolpidem interactions to avoid appeared first on The Checkup.
The Checkup by Singlecare
DECEMBER 6, 2023
The FDA has not approved Tamsulosin for use in women and children, though at times, medical professionals may use the drug off-label in these populations for other indications. However, if it is almost time for your next dose, skip the missed dose. Do not double up. If you feel faint or fall, seek help immediately.

The Checkup by Singlecare
SEPTEMBER 15, 2023
Read the label to ensure you buy yogurt containing live active cultures. National Institute of Diabetes and Digestive and Kidney Diseases (2016) How to Prevent Diarrhea While You Take Antibiotics. Amin also suggests eating yogurt for the following reasons: It contains probiotics. It can soothe gut inflammation.

The FDA Law Blog
FEBRUARY 21, 2024
This amendment marks the first significant revision of Part 820 since 1996, which established the Quality System (QS) regulation and “included requirements related to the methods used in, and the facilities and controls used for, designing, manufacturing, packaging, labeling, storing, installing, and servicing of devices intended for human use.”
The Checkup by Singlecare
OCTOBER 16, 2023
Be sure to read food packaging labels, and avoid adding table salt to your foods. “If an allergic reaction is the cause [of swelling], Benadryl ( diphenhydramine ) can reduce itching, swelling, and progression of symptoms.” Avoid salt Salt causes the body to retain water, so when you have swelling, watch your sodium intake.

The Checkup by Singlecare
FEBRUARY 2, 2024
According to a study published in 2016 in the journal Environmental Toxicology and Pharmacology , chlorophyllin showed some promise in targeting non-small cell lung cancer cells in mice. Another study published in 2016 also found that chlorophyll may be helpful for people with anemia. Pharmacopeia , UL Solutions , and Consumer Lab.

The FDA Law Blog
JANUARY 18, 2024
FDA conducted the eight-factor scheduling analysis required by the CSA in 2016 and found that marijuana continued to meet the scheduling criteria for remaining in schedule I. 12, 2016); Denial of Petition to Initiate Proceedings to Reschedule Marijuana, 81 Fed. Denial of Petition to Initiate Proceedings to Reschedule Marijuana, 81 Fed.
The Checkup by Singlecare
JANUARY 18, 2024
Carvedilol is also used off-label for multiple other indications, like angina or chest pain, atrial fibrillation , early treatment and secondary prevention of heart attacks, left ventricular dysfunction, and ventricular tachycardias. Food and Drug Administration (FDA) to treat congestive heart failure and hypertension.

The Thyroid Pharmacist
SEPTEMBER 6, 2023
Personal Care : The Wellnesse line of haircare and toothpastes by Katie Wells (aka the “Wellness Mama”) contains high-quality, safe ingredients that are clearly labeled, so there are no surprises! Published 2016 May 24. (My readers can use code IZABELLAWENTZ for 15% off all Starter Kits.) 2010;20(7):755-761. doi:10.1089/thy.2010.1636

IDStewardship
MARCH 12, 2023
It’s from 2016 about the future of antibiotics and resistance, but it remains highly relevant today and people consistently give positive feedback about it. ” Labeling the use of an antibiotic as inappropriate or appropriate cannot simply be done based upon whether it is FDA-approved for a given indication.

The Thyroid Pharmacist
OCTOBER 7, 2022
Sure enough, she read all the labels on her supplements and in her pantry, and gave her kitchen a stevia-free makeover. I encourage you to read the labels and look for 100% pure, USDA-approved stevia products where possible. doi:10.1155/2016/9132052. She also stopped baking with stevia — and was able to sleep like a baby!

PharmaShots
MARCH 9, 2023
In 2016, she received a special career-recognition award from Fundamed Foundation (a non-profit Spanish foundation) in the category of the most influential pharmaceutical executive from Spain over the past 15 years Education: Belén is an alumnus of the University of Alcala de Henares as an MS, Ph.D.,
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content