Manufacturing Execution Systems & Electronic Production Records Management: A Building Block for Pharma 4.0™ & Your Organisation’s Digitlization Journey
ISPE
JUNE 15, 2023
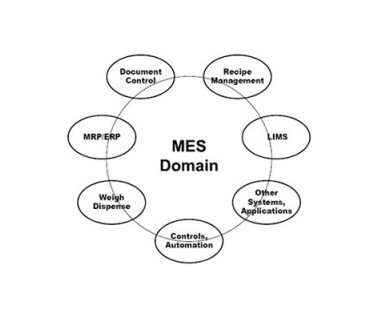
Manufacturing Execution Systems & Electronic Production Records Management: A Building Block for Pharma 4.0™ ™ & Your Organisation’s Digitlization Journey Trudy Patterson Thu, 06/15/2023 - 09:27 iSpeak Blog iSpeak Manufacturing Execution Systems & Electronic Production Records Management: A Building Block for Pharma 4.0™





































Let's personalize your content