STAT+: A nonprofit does deals in Brazil and India to make low-cost CAR-T cell therapies widely available
STAT
MAY 8, 2024
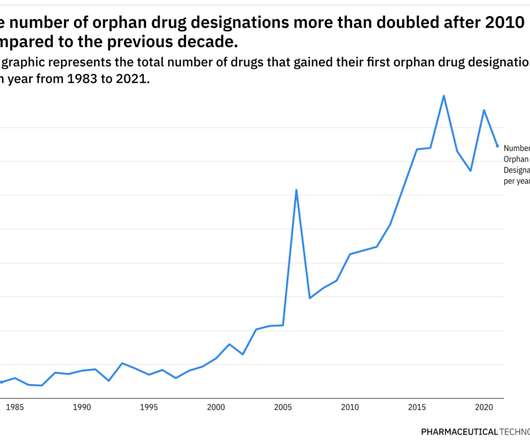
In the latest bid to widen access to medicines, a nonprofit is sending technology and materials for making expensive CAR-T cell therapies to the Brazilian government and an Indian manufacturer that will, in turn, look to provide the treatments available at a fraction of current prices in the U.S.






































Let's personalize your content