STAT+: CMS will use outcomes-based agreements in bid to help Medicaid pay for sickle cell gene therapies
STAT
JANUARY 30, 2024
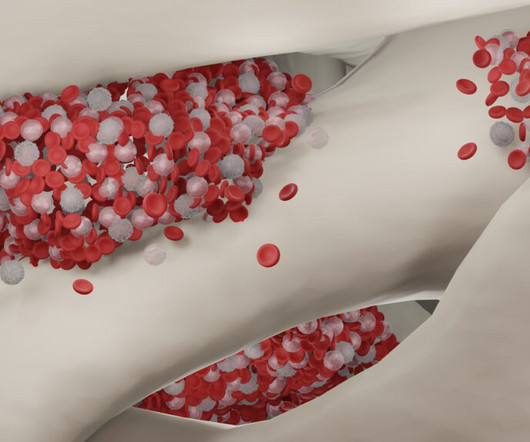
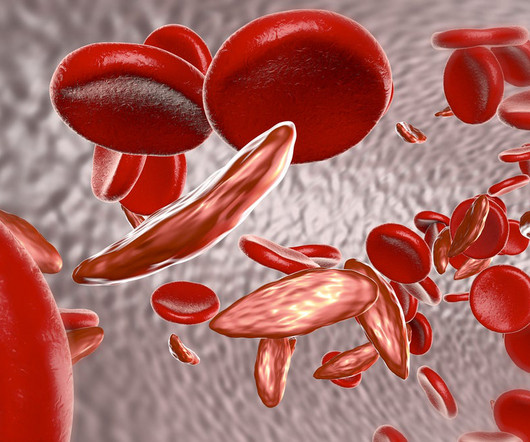
In response to concerns over multimillion-dollar price tags for new gene therapies for sickle cell disease, the U.S. Sickle cell disease affects an estimated 100,000 Americans. Two therapies recently approved by health regulators cost $2.2 million and $3.1 million and $3.1



















Let's personalize your content