BREAKING: Breast cancer drug licensed as preventative measure
Outsourcing Pharma
NOVEMBER 7, 2023
A breast cancer drug that has been used for many years to treat the disease, has been licensed today (November 7) as a preventative measure.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

 measure-licensing-use
measure-licensing-use 
Outsourcing Pharma
NOVEMBER 7, 2023
A breast cancer drug that has been used for many years to treat the disease, has been licensed today (November 7) as a preventative measure.

STAT
APRIL 24, 2023
Marburg could soon become the second virus in the past year to have experimental vaccine candidates ready for testing under an emergency use listing. Until this point, no licensed vaccines or treatments are available for Marburg.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

European Pharmaceutical Review
OCTOBER 31, 2022
Patients with a history of heart disease or psychiatric disorders were found to use amfepramones, heightening their risk of these conditions. The committee also found evidence of patients using amfepramones during pregnancy, putting their unborn infants in danger too. Earlier regulatory reviews of amfepramone products.

European Pharmaceutical Review
OCTOBER 24, 2022
Adaflex, the UK’s first melatonin product for six to 17 year-olds diagnosed with ADHD and insomnia (where sleep hygiene measures have been inadequate), is now available in the UK. Melatonin is commonly used to treat insomnia. However until now, prescribing a treatment for children with ADHD has been off-label or unlicensed in the UK.

pharmaphorum
AUGUST 18, 2021
Akili already has the first and only FDA cleared video game-based digital therapeutic (DTx) for children with attention-deficit hyperactivity disorder (ADHD), but is looking to add to its range via a licensing deal with Australia’s TALi Digital. . The effects have been shown to last for at least three months after a course is completed.

pharmaphorum
SEPTEMBER 12, 2021
Despite this, the field hasn’t been a big adopter of digital health technologies, and the pandemic has been a real catalyst for change, helped by relaxation of regulations over the use of smartphone apps like Apple FaceTime, Google Duo and Zoom for consultations.

European Pharmaceutical Review
JULY 20, 2022
1 But while data on adverse reactions is rightly used to help with these decisions, there is currently no additional modelling on the positive impact regulatory action could have on public health. Monitoring and studying the side effects of licensed medicines is an essential part of drug development to ensure public safety.
pharmaphorum
NOVEMBER 17, 2022
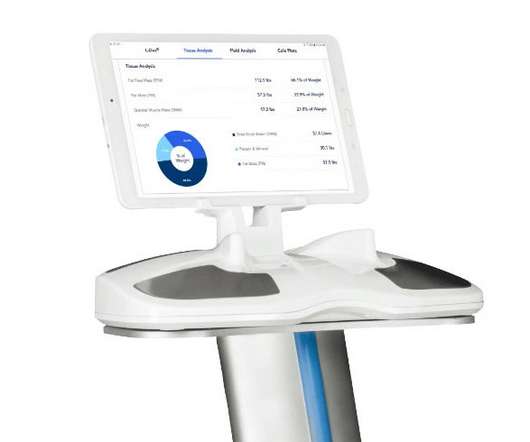
AstraZeneca has extended a contract to use a digital health device developed by ImpediMed that is being deployed in a trial of two of its drugs in chronic kidney disease (CKD). SOZO measures 256 data points over a spectrum of frequencies from 3 kHz to 1000 kHz, with results available immediately online to allow sharing.
pharmaphorum
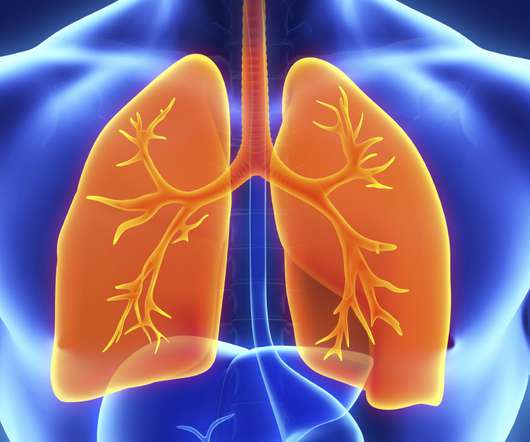
APRIL 14, 2022
ResApp’s technology was created to detect a wide range of respiratory conditions such as pneumonia, asthma, bronchiolitis and chronic obstructive pulmonary disease (COPD) using only the sound of a patient’s cough.
PQA
AUGUST 11, 2023
Learn more about our commitment to standardized pharmacy measures below and how you can connect with us next week!We If you have news you'd like for us to spotlight in an upcoming Five For Friday, send us an email! The registration link is in the Member Resources Library. Learn more about this year's Leadership Summit.

Pharmaceutical Technology
APRIL 13, 2023
ImaginAb will be responsible for licensing and supplying the clinical doses of 89Zr crefmirlimab berdoxam, its investigational CD8 ImmunoPET tracer, to Leucid Bio. Leucid Bio will use the CD8 ImmunoPET tracer along with LEU011 targeting NKG2DL, autologous CAR T-cells, in its basket study to treat solid tumours.

pharmaphorum
JULY 16, 2021
French drugmaker Ipsen has made another foray into the Parkinson’s disease category, licensing rights to an oral dopamine D3 receptor antagonist from Sweden’s IRLAB for $28 million upfront. . It is due to readout in the first half of next year.

Hospital Pharmacy Europe
AUGUST 17, 2023
Cipaglucosidase alfa is a long-term enzyme replacement therapy (ERT) used in combination with the enzyme stabiliser miglustat for adults with late-onset Pompe disease. This rare, inherited lysosomal disorder is caused by deficiency of the enzyme acid alpha-glucosidase (GAA).

pharmaphorum
NOVEMBER 16, 2021
In the second of a two-part series, Leela Barham argues that whilst international comparisons of uptake are useful, there could be more to gain from looking at more medicines and their uptake in England rather than looking at a small set of medicines and their uptake internationally. Broaden the scope of work to more medicines.
European Pharmaceutical Review
NOVEMBER 3, 2022
EMA’s Committee for Medicinal Products for Human Use (CHMP) decides the product is of high importance for public health and therapeutic innovation. It combines GSK’s proprietary AS01E adjuvant, which contains QS-21 Stimulon adjuvant licensed from Antigenics Inc. Accelerated assessment?is is offered to MAAs if the?EMA’s

European Pharmaceutical Review
MARCH 1, 2023
Three-dimensional images using computed tomography (CT) provide a valuable, non‑invasive insight into the inner morphology of freeze-dried products. While traditional optical methods can only assess the surface, X-ray technology can be used to display the internal structure, and thus may detect defects and deviations in geometry.

European Pharmaceutical Review
NOVEMBER 2, 2023
Short-term measures to take control of current shortages in addition to longer-term, preventative actions have been part of the industry’s collective response. They could provide a temporary replacement for potentially unavailable, essential licensed medicines, the EDQM explained in its statement.

pharmaphorum
JUNE 17, 2021
Tau is a protein that is found in cells of the central nervous system and is involved in the assembly and stabilisation of neuronal microtubules – channels used to transport substances to different parts of the nerve cell. In Alzheimer’s, the protein runs amok, forming tangles that have been linked to cell damage and neuronal death.
Impact Pharmaceutical Services
MAY 4, 2022
Abbreviations: BLA= Biologics License Application; IND= Investigational New Drug; NDA= New Drug Application; RMAT= Regenerative Medicine Advanced Therapy. *A A clinically significant or meaningful endpoint directly measures how a patient feels, functions, or survives and represents or characterizes the clinical outcome of interest.

pharmaphorum
FEBRUARY 24, 2021
US biotech Day One has taken ownership of a cancer drug that has been languishing in Merck KGaA’s pipeline for years, in the hope of giving it a new lease of life. . The financial details of the Merck licensing deal haven’t been made public. The financial details of the Merck licensing deal haven’t been made public.

The People's Pharmacy
JUNE 12, 2023
Several readers have written about using the sugar remedy in such cases. This old-fashioned approach may not work miracles, but many people praise its usefulness. If I ordered that mixture for a patient today, I’d lose my license. But I did use it on my mother and the decubiti cleared in a week. It worked.

pharmaphorum
OCTOBER 27, 2022
Santhera has completed a rolling application for its Duchenne muscular dystrophy (DMD) therapy vamorolone in the US, setting up a possible approval and launch in the latter half of 2023. The post Santhera seeks speedy FDA review of Duchenne drug vamorolone appeared first on.
Druggist
AUGUST 3, 2020
Otrivine Extra Dual Relief indicated use Otrivine Extra spray is used for the management of congestion and rhinorrhoea caused by colds. When used locally, for example, application inside the nose, ipratropium bromide stops secretions from the glands located in the lining of the nose (Bausch & Lomb, 2016).

European Pharmaceutical Review
OCTOBER 12, 2023
Manfrin told EPR that a key challenge in developing the scheme was “ensuring a robust process for measuring the scheme’s success. Manfrin told EPR that a key challenge in developing the scheme was “ensuring a robust process for measuring the scheme’s success.
pharmaphorum
JUNE 6, 2022
months – a result that AZ said is “potentially redefining treatment for approximately half of all patients with breast cancer” It’s a spectacular result in a patient population with few effective treatment options and the first clear evidence that anti-HER2 therapy can be useful in a much broader range of patients than at present.
Pharmaceutical Technology
OCTOBER 14, 2022
The two companies will aim to improve the manufacturing and analytic procedures used to develop personalised cell and gene therapies to treat cancer patients. Xcellbio said that the AVATAR AI technology uses the tight environmental control of the AVATAR product family.

The FDA Law Blog
DECEMBER 7, 2022
Valentine — On November 22, 2022, FDA approved CSL Behring’s BLA for Hemgenix (etranacogene dezaparvovec), an AAV-based gene therapy for the treatment of adults with Hemophilia B who currently use Factor IX prophylaxis therapy, have current or historical life-threatening hemorrhage, or have repeated, serious spontaneous bleeding episodes.

pharmaphorum
JANUARY 5, 2023
Addiction treatment specialist Spero Health has expanded an agreement to use Pear Therapeutics’ prescription digital therapeutics (DTx) in its network of US clinics. We believe having access to patient-reported data via Pear’s clinician dashboard gives us better insight into patient behaviours.”.
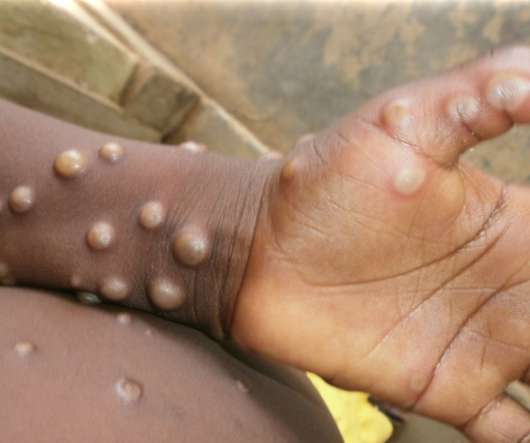
pharmaphorum
MAY 24, 2022
Meanwhile, attention is now being turned to other measures to control the outbreak, including the use of vaccines against smallpox – a related virus – in a ‘ring vaccination’ approach designed to control the spread among contacts. There’s also a licensed antiviral drug for monkeypox.

European Pharmaceutical Review
JANUARY 25, 2023
DEG and EG are toxic chemicals used as industrial solvents and antifreeze agent. Ensure that all medical products in their respective markets are approved for sale by competent authorities and obtainable from authorised/licensed suppliers in the supply chain. Most are young children under the age of five.
pharmaphorum
NOVEMBER 10, 2022
Annual checkups, cancer screenings, and vaccines are just some of the preventative measures that can help save lives, reducing the need for treatment and reliance on potentially costly medicines that are in short supply. Though often used interchangeably, there’s a key difference between the two terms. Preventive vs. diagnostic care.

Pharmaceutical Technology
JUNE 7, 2023
Rocuronium is a widely used intermediate-acting neuromuscular blocker. Baudax Bio also reported that additional data measured by electromyography (EMG) showed BX1000’s comparableness to rocuronium. All three agents are licensed from Cornell University, New York. mg/kg administered intravenously.
Pharmaceutical Technology
APRIL 19, 2023
While the Clarksville, US-based company completed a Phase Ia study in healthy participants to eventually develop the drug for Alzheimer’s disease, it is likely that the company will now focus on Parkinson’s disease in its Phase II study first, said Kelleher-Andersson. Similarly, research collaborations are also a consideration.

pharmaphorum
SEPTEMBER 20, 2021
The answer is probably, but to stop using them entirely would be short-sighted. Similar to how the advertising industry has shifted to using digital tools rather than older methods of mass marketing such as newspapers and tv commercials, personalisation is key to the future of pharma sampling. “An Personalisation is Key.

European Pharmaceutical Review
JULY 13, 2023
1,2 Yet the pace of adoption in Europe has notably lagged countries such as Canada, Australia and the US. 1 In many countries, medical cannabis is only utilised as a therapy when licensed medications have proven ineffective. The European Cannabis Report (7th Ed.). Expert Review of Clinical Pharmacology. 2023 Mar 4;16(3):257-66.
Pharmaceutical Technology
MARCH 9, 2023
According to GlobalData’s Technology Foresights, which uses over 756,000 patents to analyse innovation intensity for the pharmaceutical industry, there are 110 innovation areas that will shape the future of the industry. Immatics has established two proprietary cancer cell target and TCR discovery platforms, namely XPRESIDENT and XCEPTOR.

pharmaphorum
DECEMBER 2, 2021
It will focus its efforts initially on neurological diseases like Alzheimer’s and Parkinson’s, using technologies that can measure how disease alters tissues, cells and proteins, with the aim of increasing the success rate of drug discovery and development. billion to the pot.
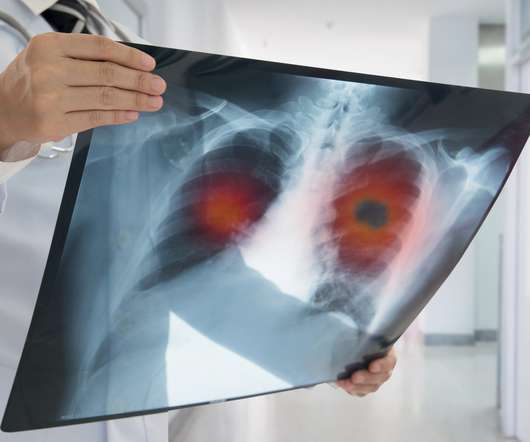
pharmaphorum
OCTOBER 19, 2021
Eli Lilly made a late entry into the checkpoint inhibitor market when it licensed ex-China rights to Innovent’s sintilimab last year, and a new trial in lung cancer will raise its hopes of getting a good return on its investment.

The FDA Law Blog
FEBRUARY 2, 2023
While this hype may be warranted in some respects—a 60-year old legal provision has now been amended to acknowledge that the science of drug development is advancing—the change is mostly symbolic and is likely to take many years before we see it have a measurable impact. 42 U.S.C. § 262(k)(2)(A)(i)(I).
pharmaphorum
NOVEMBER 30, 2021
Eisai has licensed rights to a cognition-checking algorithm developed by Australian digital health company Cogstate, which it uses in an app – called NouKNOW – that allows people to check their brain health. Phone users will be provided with BPI checks at no cost using the app up to four times a year.
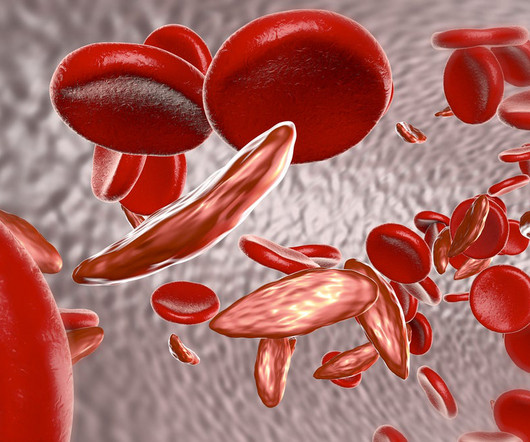
Pharmaceutical Technology
APRIL 13, 2023
Released on April 12, the report focuses on bluebird bio’s lovotibeglogene autotemcel and Vertex Pharmaceuticals and CRISPR Therapeutics’ exagamglogene autotemcel or exa-cel and their potential use in treating sickle cell disease. Bluebird already uses a lentiviral vector in its approved gene therapy for beta-thalassemia called Zynteglo.

pharmaphorum
MAY 30, 2022
RhoC overexpressed in cancer cells that are prone to spreading compared with healthy cells across multiple cancer types, raising the hope that RV001 could be a broadly useful, tumour agnostic treatment for cancer.
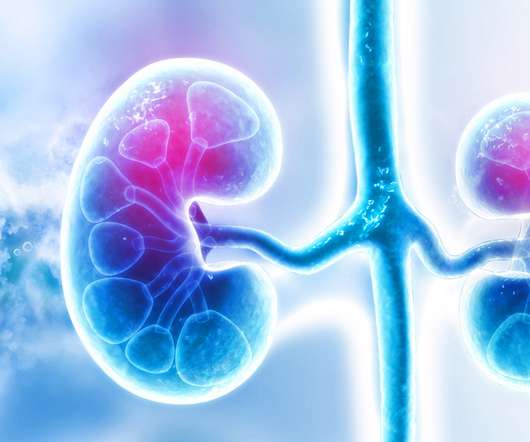
pharmaphorum
JULY 29, 2022
ESRD tend to have underlying conditions like diabetes, high blood pressure, atrial fibrillation, and cardiovascular disease that increase their risk of blood clots, and systemic anticoagulants are routinely used as a preventative measure. However, the high rates of haemorrhage make the treatment challenging.

European Pharmaceutical Review
DECEMBER 21, 2022
Since then, there have been few breakthrough innovations in treating neuropsychiatric diseases such as anxiety, depression, substance use disorders (SUDs), and post-traumatic stress disorder (PTSD). This EU pathway is typically used when sponsors want to broaden a therapeutic’s indications, but regulators require more data.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content