Tumour-infiltrating lymphocyte therapy in advanced melanoma superior to ipilimumab
Hospital Pharmacy Europe
SEPTEMBER 28, 2022
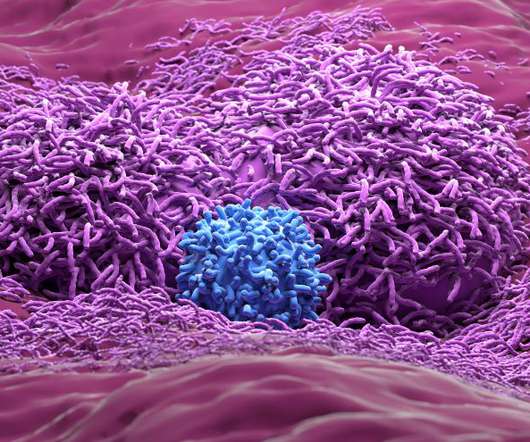
Tumour-infiltrating lymphocyte therapy (TILT) gave rise to superior progression-free survival in comparison to immune checkpoint inhibitor therapy with ipilimumab in a phase 3 trial of patients with advanced melanoma according to the results of a study presented at the European Society for medical Oncology (ESMO) Congress by Dutch researchers.


































Let's personalize your content