Rising Demand for Pharmaceutical Secondary Packaging Providers
Roots Analysis
APRIL 20, 2022
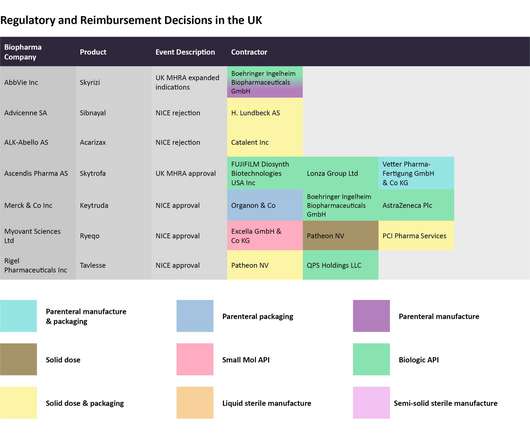
On an average, around 50 drugs are approved by the US Food and Drug Administration (US FDA) annually. Further, studies indicate that more than 100,000 tons of pharmaceutical products are consumed globally per year. 3] Some of the advantages offered by pharmaceutical secondary packaging have been depicted below.




































Let's personalize your content