Sandoz Launches Action Plan to Increase Global Biosimilar Access
Drug Topics
JUNE 22, 2023
The Act4Biosimilars Action Plan aims to highlight key challenges preventing patient access to biosimilars and outline steps to help overcome them.

Drug Topics
JUNE 22, 2023
The Act4Biosimilars Action Plan aims to highlight key challenges preventing patient access to biosimilars and outline steps to help overcome them.

PhRMA
JUNE 22, 2023
Research doesn’t end once a medicine is first approved by the U.S. Food and Drug Administration (FDA). Researchers often spend years after an initial approval exploring and obtaining approval of new indications for medicines to treat other patient populations desperate for new treatment options. Patients with cancer or rare diseases often depend on this post-approval research.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Drug Topics
JUNE 22, 2023
A study found that pharmacists possessed moderate to low knowledge scores when it came to statements tackling biosimilars.

Pharmacy Times
JUNE 22, 2023
Treatment with lisocabtagene maraleucel led to strong and durable response rates with little serious adverse effects in patients with relapsed or refractory follicular lymphoma and mantle cell lymphoma.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.

Fierce Healthcare
JUNE 22, 2023
Physician-owned general acute care hospitals charge less than other non-physician-owned facilities in their region for several common shoppable care services, according to a new analysis published | A new analysis of competing hospitals' published prices adds fuel to the debate over whether Congress should lift the Affordable Care Act's ban on new physician-owned hospitals.

Fierce Pharma
JUNE 22, 2023
Even after second application attempt, Intercept’s Ocaliva wasn’t the fatty liver disease breakthrough that the company had hoped it would be. | Even after second application attempt, Intercept’s Ocaliva wasn’t the fatty liver disease breakthrough that the company had hoped it would be. Intercept is now swinging into restructuring mode, and the NASH baton passes on to Madrigal Pharma.
Pharmacy Technician Pulse brings together the best content for pharmacy technicians from the widest variety of industry thought leaders.
STAT
JUNE 22, 2023
When I was diagnosed with Duchenne muscular dystrophy 20 years ago, there was no hope. The guidance the diagnosing doctor gave my parents was simple: Love your child as much as you can now because he won’t be here very long. That was the inspiration that my mom needed to start CureDuchenne. Her intention — our whole family’s intention — was to cure this disease so no other parents would have to go through the same traumatic prognosis.

Pharmacy Times
JUNE 22, 2023
The Act4Biosimilars Action Plan highlights major challengers in preventing access to biosimilars and actionable steps to accelerate the adoption by overcoming these challenges.

Fierce Healthcare
JUNE 22, 2023
Ochsner Health and The University of Texas MD Anderson Cancer Center are partnering to build an integrated cancer care program in southeastern Louisiana, the organizations announced Thursday. | The first phase of the new collaboration will see cancer patients at seven of Ochsner's southeast Louisiana facilities become eligible for breaking clinical trials.

Pharmacy Times
JUNE 22, 2023
Christina Madison, PharmD, FCCP, AAHIVP, sat down with Sandra Leal, PharmD, MPH, FAPhA, CDCES, vice president of pharmacy practice innovation and advocacy at CVS Pharmacy, to discuss how pharmacists are diving into public health and expanding their roles.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

Fierce Pharma
JUNE 22, 2023
The EMA is scrutinizing GLP-1s, raising a safety signal about the risk that drugs from Novo Nordisk, Eli Lilly and other companies could cause cancer.

Pharmacy Times
JUNE 22, 2023
Extended use of an N95 mask in daily life can cause various cardiopulmonary stresses, such as reduced respiration, elevated heart rate and blood pressure, and increased energy expenditure.

Fierce Pharma
JUNE 22, 2023
The Centers for Disease Control and Prevention's (CDC's) Advisory Committee on Immunization Practices (ACIP) has recommended that adults 60 and older—in consultation with their doctors—receive vacc | The CDC's Advisory Committee on Immunization Practices has recommended that adults 60 and older—in consultation with their doctors—receive vaccines to prevent lower respiratory tract disease caused by respiratory syncytial virus.

Pharmacy Times
JUNE 22, 2023
As part of the approval condition, the FDA is requiring Sarepta Therapeutics to complete a clinical study to confirm the drug’s benefit in motor and physical functions.

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.

STAT
JUNE 22, 2023
The Food and Drug Administration granted conditional approval Thursday to the first gene therapy for Duchenne muscular dystrophy. Regulators restricted the treatment to younger patients, with additional data required to broaden its use. The gene therapy, called Elevydis, is made by Sarepta Therapeutics. The company will charge $3.2 million for the treatment, making it the U.S.
Pharmacy Times
JUNE 22, 2023
The approval was based on data from 2 studies of blinatumomab in adults and pediatric patients with CD19-positive B-cell precursor acute lymphoblastic leukemia.
STAT
JUNE 22, 2023
Pfizer announced last week that it expects to run out of a key drug for treating syphilis in the near future — a looming problem that health professionals say could exacerbate syphilis rates, widen racial disparities in sexually transmitted diseases, and stymie global access to the antibiotic, especially within lower-income countries. The drug in question is Bicillin, an injectable, long-acting form of penicillin most commonly used to treat syphilis in adults as well as childhood infectio

Pharmacy Times
JUNE 22, 2023
Health Mart is offering a new basic training program with ASHP/ACPE accreditation for pharmacy technicians, along with accredited and non-accredited training.

STAT
JUNE 22, 2023
WASHINGTON — The Food and Drug Administration is ramping up its efforts to force vape shops and other retailers to stop selling unauthorized disposable vape brands that are increasingly popular with young people. The FDA recently issued 189 formal warning letters to shops selling two brands of vape products, Elf Bar and Esco Bars, the agency announced in a press release Thursday.

Pharmacy Times
JUNE 22, 2023
Six-month preexposure prophylaxis (PrEP) dispensing supported by HIV self-testing resulted in PrEP adherence after 12 months and less visits to clinical sites.
Drug Topics
JUNE 22, 2023
In a large observational study, significant information was obtained about mRNA vaccine protection against acute infection and disease severity.

Pharmacy Times
JUNE 22, 2023
Utilizing longitudinal assessments of language impairment and clinical progression could help identify challenges earlier.

STAT
JUNE 22, 2023
The number of people with diabetes worldwide is set to more than double to 1.3 billion by 2050, a new study finds, a trend accelerated by widening inequities both between and within countries. By 2050, about 1 in 10 people around the world are predicted to have the disease, representing a 60% surge in the prevalence of diabetes, according to the study, published Thursday in the Lancet as part of a wide-ranging series on global inequities in diabetes.
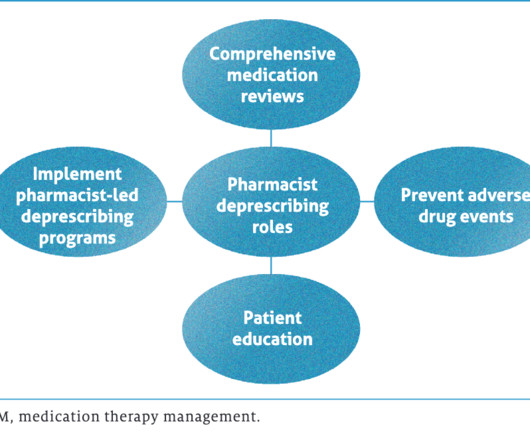
Pharmacy Times
JUNE 22, 2023
Pharmacists play key roles in performing comprehensive patient reviews, reducing polypharmacy.

Fierce Healthcare
JUNE 22, 2023
The Boys & Girls Clubs of America (BGCA) is on the front lines of battling the youth mental health crisis, and it's securing reinforcements in the form of a $10 million grant from the Blue Cros | The Blue Cross Blue Shield Association hopes that its $10 million grant to the Boys & Girls Clubs of America will play a part in addressing the mental health crisis facing America’s youth.

STAT
JUNE 22, 2023
Medical device makers may soon have an easier path to securing health insurance reimbursement for their products, following the notice Thursday of a long-awaited rule by the Centers for Medicare and Medicaid Services. The program , called Transitional Coverage for Emerging Technologies, would apply to medical products deemed “breakthrough devices” by the Food and Drug Administration.

Hospital Pharmacy Europe
JUNE 22, 2023
An electronic prescribing (EP) system within an intensive care unit (ICU) requires a lower level of clinical pharmacist input compared to a paper-based system, according to a recent prospective, longitudinal UK study. Although EP systems are designed to reduce medication errors , the quality of evidence on their effectiveness is variable. Nevertheless, within an ICU setting, the use of commercial computerised provider order entry systems led to an 85% reduction in medication prescribing error ra
STAT
JUNE 22, 2023
Over 1,000 times a day, distressed people call crisis support lines operated by Protocall Services. Its counselors are carefully trained for the sensitive and taxing conversations, but even with supervision on the job, major errors, like failing to screen for suicide, can go undetected. So Portland, Ore.-based Protocall is working with a company called Lyssn to investigate if technology can help keep call quality high.

Drug Store News
JUNE 22, 2023
Foster & Thrive is intended to help meet the evolving needs and growing demand for quality, over-the counter private-label health and wellness products.
STAT
JUNE 22, 2023
WASHINGTON — The health care system is on the precipice of broad access to a treatment for Alzheimer’s for the first time — and it’s scrambling to figure out how to handle it. Right now, most people with mild cognitive impairment who would qualify for Eisai and Biogen’s drug Leqembi are in the Medicare program, which has restricted which patients can receive the medication to those who are enrolled in clinical trials.

Drug Store News
JUNE 22, 2023
The first version of the framework introduced by Albertsons Media Collective concentrates on four areas: product characteristics, performance measurement, third-party verification and capabilities.

Fierce Pharma
JUNE 22, 2023
In this episode of "The Top Line," we talk with Fierce’s Eric Sagonowsky and Kevin Dunleavy about the most expensive drugs in the U.S. | This week on "The Top Line," we discuss the most expensive drugs in the U.S., plus Eli Lilly's recent acquisition, the latest company to challenge the Inflation Reduction Act, and the rest of the week's headlines.

ISPE
JUNE 22, 2023
A Busy Few Months for ISPE’s Women in Pharma® Trudy Patterson Thu, 06/22/2023 - 15:21 iSpeak Blog iSpeak A Busy Few Months for ISPE’s Women in Pharma® Edyna Miguez 22 June 2023 ISPE’s Women in Pharma ® group advocates for accelerated equality in the pharmaceutical industry and beyond. Through virtual and in-person programming, Women in Pharma provides ISPE members the resources necessary to challenge the status quo and make an impact on both a personal and professional level.
Fierce Pharma
JUNE 22, 2023
AstraZeneca has reportedly drafted a contingency plan to spin out its China business amid increasing geopolitical risks. | AstraZeneca has reportedly worked out a contingency plan to spin out its China business amid increasing geopolitical risks. The British pharma is still forming collaborations in the country, the latest being an R&D pact focused on hypercholesterolemia.
Let's personalize your content