Adaptation Is the Key to Success
Drug Topics
MARCH 21, 2024
Farmers and welders have adapted to work with the current times. Pharmacists should consider adapting their profession as well.

Drug Topics
MARCH 21, 2024
Farmers and welders have adapted to work with the current times. Pharmacists should consider adapting their profession as well.
STAT
MARCH 21, 2024
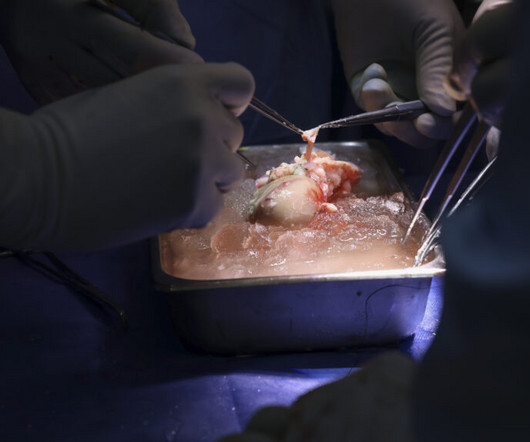
In a new test of xenotransplantation, a medical team at Massachusetts General Hospital announced Thursday that, for the first time, it had transplanted a kidney from a CRISPR gene-edited pig into a living patient. Surgeons performed the milestone procedure over four hours on Saturday, March 16, without complications. As of Thursday morning, the organ recipient, a 62-year-old man named Richard Slayman, was “recovering well” and expected to be discharged soon, the hospital said in a
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Drug Topics
MARCH 21, 2024
Researchers collected data to measure inequities in pharmacy access across the country.

Fierce Healthcare
MARCH 21, 2024
More than 100 provider organizations want the Centers for Medicare & Medicaid Services (CMS) to take a tougher stance on Medicare Advantage (MA) plans’ practices following an industry survey es | Providers spent nearly $20 billion in 2022 pursuing delays and denials across all payer types, yet those efforts are substantially more costly on average when dealing with private plans, hundreds of hospitals and health systems told Premier in a recent survey.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.

Drug Topics
MARCH 21, 2024
Rilpivirine (Edurant Ped) effectively suppresses the HIV-1 virus in pediatric patients with HIV-1 RNA less than or equal to 100,000 copies/mL in combination with other antiretroviral therapies.

STAT
MARCH 21, 2024
Early data regarding the use of GLP-1 medications like Ozempic and Wegovy to treat addiction is “very, very, exciting,” Nora Volkow, the director of the National Institute on Drug Abuse, said Thursday. But even as she expressed enthusiasm for the new drugs’ potential, Volkow criticized pharmaceutical companies for neglecting a moral imperative to develop new addiction treatments — but acknowledged that the health system more broadly doesn’t incentivize drug com
Pharmacy Technician Pulse brings together the best content for pharmacy technicians from the widest variety of industry thought leaders.

STAT
MARCH 21, 2024
CRISPR is no longer a promising but unproven technology — it is a reality. But for this powerful gene-editing tool to reach its full potential, researchers and disease advocates say they’ll have to solve a thorny problem: connecting patients suffering from devastating diseases with therapies that could help them. Experts pointed to lab-developed tests, or LDTs, as one important element that could help make this connection during a panel at the STAT Breakthrough Summit East in New Y

Pharmacy Times
MARCH 21, 2024
The data comes from 2 phase 4 trials, ENABLE and EMPOWER, which evaluated lanadelumab and the outcomes of treatment in adolescents with hereditary angioedema.
STAT
MARCH 21, 2024
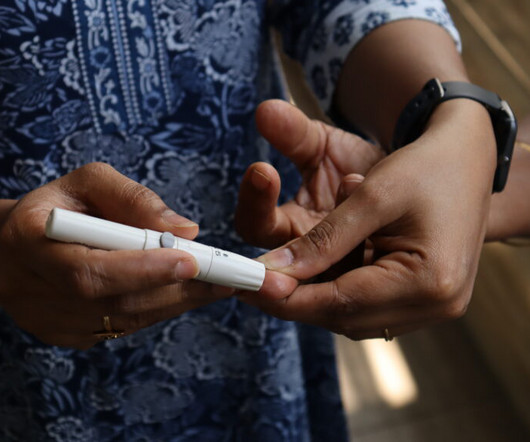
The last decade has seen billions of dollars flow into digital health companies that promise to improve outcomes for the 38 million Americans living with type 2 diabetes. Their products aren’t cheap, but in the long term, they pitch to health plans and employers that these digital tools will help cut health care costs by preventing serious complications like amputation and kidney failure.

Fierce Healthcare
MARCH 21, 2024
Chipmaker Nvidia sees big opportunities in healthcare AI and this week it launched more than two dozen new gen AI-powered microservices. | The global computing powerhouse inked collaborations with Abridge, Microsoft, GE HealthCare and Hippocratic AI to to expand its generative AI capabilities into healthcare and life sciences.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

STAT
MARCH 21, 2024
The psychedelic ibogaine is unlikely to ever receive approval as a treatment for opioid addiction, the federal government’s top addiction researcher said Thursday. The remarks from Nora Volkow, the longtime director of the National Institute on Drug Abuse, serve as a cautionary note amid widespread enthusiasm about ibogaine, a naturally occurring substance that drug companies and researchers have increasingly cast as a potential paradigm-shifting addiction treatment.
Pharmacy Times
MARCH 21, 2024
The FDA has only approved aprocitentan (Tryvio; Idorsia) in the 12.5 mg dosage as the effects were similar between the 25 mg and 12.5 mg dosages.

STAT
MARCH 21, 2024
George Yancopoulos is in touch with his teenage self. After 35 years as head scientist at Regeneron Pharmaceuticals, Yancopoulos has led the development of 12 approved medicines, turning a once-fledgling company into the $100 billion global drugmaker it is today. And you can trace a straight line back to the childhood fascination with science he fostered growing up in Woodside, Queens.

pharmaphorum
MARCH 21, 2024
There is no denying that artificial intelligence (AI) has captured the imagination of innovators working across the pharmaceutical sector. The drug discovery space is no different, with myriad applications promising to revolutionise the way that researchers and sponsors approach identifying and progressing new drug candidates.

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.

STAT
MARCH 21, 2024
As more states create dedicated boards to cap the costs of medicines, some drugmakers and their allies are pushing back with a controversial tactic — lobbying for legislation to set exemptions for so-called orphan drugs, which are used to combat rare diseases that afflict relatively small groups of patients. The efforts, which are being joined by some some lawmakers, reflect concerns that patients may lose access to these medicines if pharmaceutical companies halt sales or decide not to i

pharmaphorum
MARCH 21, 2024
On today’s podcast, host Jonah Comstock is joined by Meri Beckwith, co-founder of Lindus Health, a next-gen CRO that includes digital therapeutics among its specialties. They talk a little bit about the often unseen difficulties of validating a digital therapeutic through a clinical trial.

STAT
MARCH 21, 2024
When the first biotech companies emerged to capitalize on the revolutionary genome-editing technology CRISPR, Editas Medicine seemed like the pacesetter. It locked up some of the crucial IP, and had behind it the pioneering scientist Feng Zhang of the Broad Institute as one of its founders. But now, a decade later, Editas is seen as lagging behind its peers.
Fierce Healthcare
MARCH 21, 2024
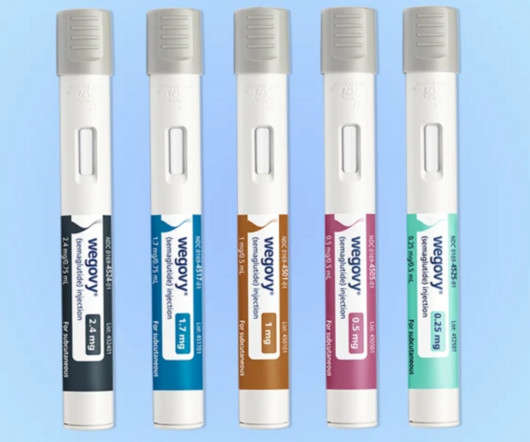
Medicare Part D may cover obesity drugs when they secure approval for additional, medically accepted uses, according to a WSJ report.
STAT
MARCH 21, 2024
What happens after you close a nearly $11 billion acquisition? Well, for Prometheus Therapeutics’ former CEO Mark McKenna, there was a brief vacation. But within weeks of the company’s acquisition by Merck closing last June, he was thinking about ideas for the future. Investors were floating ideas by him. McKenna discussed possibilities with his teenagers and Prometheus’ chief scientific officer.

Fierce Pharma
MARCH 21, 2024
Drug developers often try to spot tidbits of positive information from a failed clinical trial. | Drug developers often try to spot tidbits of positive information from a failed clinical trial. But Roche is doing the opposite for its positive Enspryng study in the competitive autoimmune disorder of myasthenia gravis.
STAT
MARCH 21, 2024
The adverse childhood experience (ACE) questionnaire has become a critical part of public health. It offers physicians a screening tool to evaluate patients, gaining valuable insights into their physical and mental well-being. This information guides the implementation of preventive measures like lifestyle counseling to reduce the risk of ACE-related chronic conditions like depression, obesity, asthma, diabetes, and cancer.

pharmaphorum
MARCH 21, 2024
The rising demand for mono-material packaging in the pharmaceutical industry due to its sustainability benefits will have a significant impact. Learn more about how this trend is shaping the future of pharmaceutical packaging.

STAT
MARCH 21, 2024
LONDON — Widespread genetic testing as well as the development of cutting-edge, customized genetic therapies have opened the door to treating many more inherited conditions than previously possible. A few recent cases, in which bespoke medicines were created for children with exceedingly rare mutations, have raised hopes for furthering the approach.
Hospital Pharmacy Europe
MARCH 21, 2024
Supporting the successful compounding of drugs with 3D printing processes has the potential to not only fulfil unmet patient needs but also drive efficiencies in hospital pharmacies. Ian Soulairol from the Institut Charles Gerhardt Montpellier at Montpellier University and pharmacist at the Department of Pharmacy of Nîmes University Hospital in France shares insights into how this technology is evolving and what it means for clinical practice now and in the future. 3D printing, or additive manuf

pharmaphorum
MARCH 21, 2024
Clasp Therapeutics has burst onto the T-cell engager (TCE) scene with $150 million in funding for a platform that aims to improve the safety of the class, which is currently gaining traction in cancer immunotherapy.
Fierce Healthcare
MARCH 21, 2024
Long-term and post-acute care (LTPAC) providers play a vital role in our health system. | Continuing to improve and coordinate post-acute care can lead to higher quality and lower costs. As such, long-term and post-acute care providers are in a position to lead the way on value-based care.

pharmaphorum
MARCH 21, 2024
A partnership between Eisai and Lifenet that aims to improve insurance cover for people with early dementia has generated its first policy, part of an effort by the pharma group to develop an ‘ecosystem’ to support patients.

Fierce Pharma
MARCH 21, 2024
Amid congressional scrutiny on U.S. | The drugmaker's commitment will start by January 1, 2025, and follows similar moves by peers Boehringer Ingelheim and AstraZeneca. Before the pledges, the companies—and Teva—caught Senate heat over high U.S. inhaler prices.
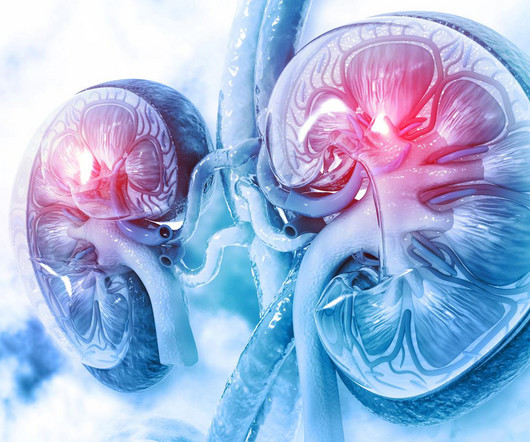
pharmaphorum
MARCH 21, 2024
Surgeons in the US have carried out the world’s first transplant of a pig kidney into a human, a feat made possible by genetic modification of the organ and an experimental immunosuppressant drug regimen to prevent rejection. The recipient, 62-year-old Richard Slayman of Weymouth in Massachusetts, had end-stage renal disease and was dependent on dialysis.

STAT
MARCH 21, 2024
I am one week away from the launch of my weekly email newsletter! It’s called Adam’s Biotech Scorecard, and it’s exclusively for STAT subscribers. One catch: We need you to sign up, which you can do here. What should you expect to see in my newsletter each week? Unfiltered, uncompromising, and (hopefully) valuable insight and analysis from the intersection of Wall Street and biotech.
Fierce Pharma
MARCH 21, 2024
Three large pharma companies plan to expand their manufacturing footprints. Kyowa Kirin’s $4.25 million gene therapy set a new world pricing record. | Three large pharma companies plan to expand their manufacturing footprints. Kyowa Kirin’s $4.25 million gene therapy set a new world pricing record. BeiGene’s PD-1 inhibitor finally crossed the FDA finish line after a 20-month delay.
STAT
MARCH 21, 2024
Want to stay on top of the science and politics driving biotech today? Sign up to get our biotech newsletter in your inbox. Hello, everyone. Damian here with yet another megaround in biotech, a preview of some hotly anticipated data, and the perils of declining revenue.

Pharmacy Times
MARCH 21, 2024
Rilpivirine (Edurant Ped; Johnson and Johnson) is indicated for the treatment of HIV in combination with other antiretroviral therapies in treatment-naïve pediatric patients.

STAT
MARCH 21, 2024
Is there a cure for allergies? Has the FDA become too flexible? And which drugs make you muscular? We cover all that and more this week on “The Readout LOUD,” STAT’s biotech podcast. Recorded live from from the STAT Breakthrough Summit East in New York City, we discuss some event highlights, including words from CRISPR pioneer Feng Zhang and Regeneron Pharmaceuticals head scientist George Yancopoulos.
Let's personalize your content