Drugmakers pledge shorter launch times for drugs in EU
pharmaphorum
APRIL 11, 2022
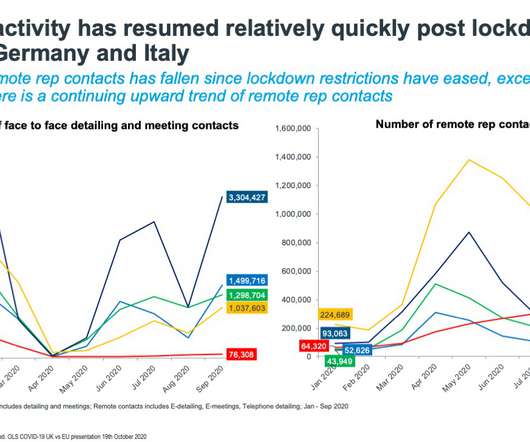
Pharmaceutical companies have said they will try to carve months off the time between approval and launch for new medicines in Europe, and reduce disparities between access between countries. — EFPIA (@EFPIA) April 11, 2022. ” The post Drugmakers pledge shorter launch times for drugs in EU appeared first on.











Let's personalize your content